![ChemChapter_4[1]Light](http://s1.studyres.com/store/data/001894151_1-323884b777914f52c04d2bb917d4088a-300x300.png)
Quantum Physics - The University of Sydney
... Describe how the Bohr model for the hydrogen atom leads to quantisation of energy and angular momentum. ...
... Describe how the Bohr model for the hydrogen atom leads to quantisation of energy and angular momentum. ...
Electronic structure and spectroscopy
... At the turning of the 19th and 20st century new experiments appeared which could not be explained by the tools of the classical (Newtonian) mechanics. For the new theory new concepts were needed: • quantization: the energy can not have arbitrary value • particle-wave dualism ⇒ development of QUANTUM ...
... At the turning of the 19th and 20st century new experiments appeared which could not be explained by the tools of the classical (Newtonian) mechanics. For the new theory new concepts were needed: • quantization: the energy can not have arbitrary value • particle-wave dualism ⇒ development of QUANTUM ...
brief answers - Inside Mines
... of discrete energy levels? What mathematical relationship describes these levels? The H spectrum is a sequence of discrete lines. Using Einstein’s hypothesis that the light carries energy hν, each line corresponds to a transition between two states of the atom. The spectrum is completely explained b ...
... of discrete energy levels? What mathematical relationship describes these levels? The H spectrum is a sequence of discrete lines. Using Einstein’s hypothesis that the light carries energy hν, each line corresponds to a transition between two states of the atom. The spectrum is completely explained b ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 3. Write the relation between group velocity and phase velocity. 4. Write the steady state form of Schrodinger’s equation. 5. What are Eigen functions and Eigen values? 6. Show that the Eigen values of Hermitian operator are real. 7. Define inertial and non–inertial frames of reference. 8. State the ...
... 3. Write the relation between group velocity and phase velocity. 4. Write the steady state form of Schrodinger’s equation. 5. What are Eigen functions and Eigen values? 6. Show that the Eigen values of Hermitian operator are real. 7. Define inertial and non–inertial frames of reference. 8. State the ...
(Bohr Model And X-Rays) Part-1
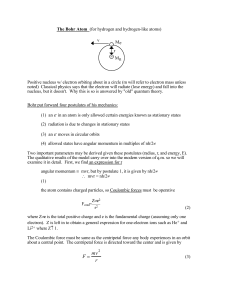
... Bohr gave following postulates for electron in hydrogen atom :• An electron in an atom could resolve in certain stable orbits without the emission of radiant energy. • An electron resolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of L= ...
... Bohr gave following postulates for electron in hydrogen atom :• An electron in an atom could resolve in certain stable orbits without the emission of radiant energy. • An electron resolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of L= ...
final2012
... b) Which is the unstable isotope and why. c) Write down the decay mechanism that converts the unstable to the stable isotope. d) Calculate the nuclear radius for these isotopes given ...
... b) Which is the unstable isotope and why. c) Write down the decay mechanism that converts the unstable to the stable isotope. d) Calculate the nuclear radius for these isotopes given ...
CH101 General Chemistry
... Schrödinger Equation: wave equation of a particle It plays a role analogous in quantum mechanics to Newton's second law in classical mechanics. The kinetic and potential energies are transformed into the Hamiltonian which acts upon the wave function to generate the evolution of the wave function in ...
... Schrödinger Equation: wave equation of a particle It plays a role analogous in quantum mechanics to Newton's second law in classical mechanics. The kinetic and potential energies are transformed into the Hamiltonian which acts upon the wave function to generate the evolution of the wave function in ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 /1.00-4.00
... 11. Discuss the failures of classical mechanics and the success of quantum theory in the explanation of black body radiation. . 12. What are quantum mechanical postulates? Explain briefly any two of the postulates. 13. The work function for Cesium is 2.14 eV. What is the kinetic energy and the speed ...
... 11. Discuss the failures of classical mechanics and the success of quantum theory in the explanation of black body radiation. . 12. What are quantum mechanical postulates? Explain briefly any two of the postulates. 13. The work function for Cesium is 2.14 eV. What is the kinetic energy and the speed ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... 16. (i) Outline the probability interpretation of the wave function. (ii) An electron has a speed of 500 m/s with an accuracy of 0.004%, calculate the certainty with which we can locate the position of the electron. iii) Can we measure the kinetic and potential energies of a particle simultaneously ...
... 16. (i) Outline the probability interpretation of the wave function. (ii) An electron has a speed of 500 m/s with an accuracy of 0.004%, calculate the certainty with which we can locate the position of the electron. iii) Can we measure the kinetic and potential energies of a particle simultaneously ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.