Franck-Hertz experiment with Ne-tube Related Topics
... Theory and evaluation Niels Bohr introduced the planetary model of the atom in 1913: An isolated atom consists of a positively charged nucleus about which electrons are distributed in successive orbits. He also postulated that only those orbits occur for which the angular momentum of the electron is ...
... Theory and evaluation Niels Bohr introduced the planetary model of the atom in 1913: An isolated atom consists of a positively charged nucleus about which electrons are distributed in successive orbits. He also postulated that only those orbits occur for which the angular momentum of the electron is ...
Atomic Structure and Periodicity
... • Extensively studied by atomic theorists such as Bohr. • High energy sparks cause hydrogen gas molecules (H-H) to break apart suddenly, with some electrons in higher energy levels than would be expected normally. • As the electrons fall back to their ground states, energy is ...
... • Extensively studied by atomic theorists such as Bohr. • High energy sparks cause hydrogen gas molecules (H-H) to break apart suddenly, with some electrons in higher energy levels than would be expected normally. • As the electrons fall back to their ground states, energy is ...
Commentary - Absurd Being
... The early Ptolemaic model of the solar system with the Earth at the centre failed on the third point so ‘epicycles’ were added to the orbits of the heavenly bodies (along with a couple of other features) to make the theory agree with observations. Although definitely unwieldy, inelegant and (as we n ...
... The early Ptolemaic model of the solar system with the Earth at the centre failed on the third point so ‘epicycles’ were added to the orbits of the heavenly bodies (along with a couple of other features) to make the theory agree with observations. Although definitely unwieldy, inelegant and (as we n ...
Photon localizability - Current research interest: photon position
... 3) Transverse fields in 3D It has long been claimed that there is no hermitian photon position operator with commuting components, and hence there is not a basis of localized eigenvectors. However, we have recently published papers where it is demonstrated that a family of position operators exists ...
... 3) Transverse fields in 3D It has long been claimed that there is no hermitian photon position operator with commuting components, and hence there is not a basis of localized eigenvectors. However, we have recently published papers where it is demonstrated that a family of position operators exists ...
No Slide Title - Rubin Gulaboski
... • de Broglie summarized the concepts of waves and particles, with noticeable effects if the objects are small. ...
... • de Broglie summarized the concepts of waves and particles, with noticeable effects if the objects are small. ...
Lamb
... of radiation - the concept of 'light quanta! completely at variance with the most fundamental concepts of the classical electromagnetic theory of radiation" ...
... of radiation - the concept of 'light quanta! completely at variance with the most fundamental concepts of the classical electromagnetic theory of radiation" ...
MIT6_007S11_lec50
... Trapping Photons: Mirrors and Waveguides How do we keep photons around for long enough time so they have a chance to stimulate an emission ? ...
... Trapping Photons: Mirrors and Waveguides How do we keep photons around for long enough time so they have a chance to stimulate an emission ? ...
Electrons and Photons
... Quantum…photon • Photon is just the name for a quantum of light • Electron Transition – when an electron moves from one level to another – When an electron transitions to a higher energy level, a photon is absorbed. ...
... Quantum…photon • Photon is just the name for a quantum of light • Electron Transition – when an electron moves from one level to another – When an electron transitions to a higher energy level, a photon is absorbed. ...
are WAVES. PARTICLES!
... The Aspect Experiment “Copenhagen” this guy’s Instant action says at a distance isn’t properties arequantum undefined until possible, so mechanics measurement happens here. must not be “complete.” ...
... The Aspect Experiment “Copenhagen” this guy’s Instant action says at a distance isn’t properties arequantum undefined until possible, so mechanics measurement happens here. must not be “complete.” ...
lecture 10
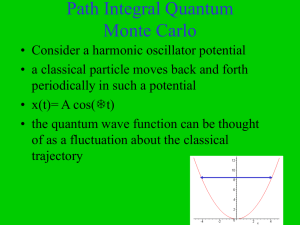
... It is clear from the above equation that particle can have only desecrated values of energies. The lowest energy that particle can have corresponds to n=o , and is called zero-point energy. It is given by ...
... It is clear from the above equation that particle can have only desecrated values of energies. The lowest energy that particle can have corresponds to n=o , and is called zero-point energy. It is given by ...
Quantum/Nuclear - Issaquah Connect
... Calculate wavelengths of spectral lines from energy level differences and vice versa ...
... Calculate wavelengths of spectral lines from energy level differences and vice versa ...
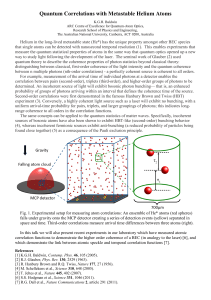
Quantum Correlations with Metastable Helium Atoms
... In this talk we will also present recent experiments in our laboratory which have measured atomic correlation functions to demonstrate the higher order coherence of a BEC (in analogy to the laser) [6], and which demonstrate the link between atomic speckle and temporal correlation functions [7]. ...
... In this talk we will also present recent experiments in our laboratory which have measured atomic correlation functions to demonstrate the higher order coherence of a BEC (in analogy to the laser) [6], and which demonstrate the link between atomic speckle and temporal correlation functions [7]. ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.