Study Guide Chap. 11
... 2. Photoelectric effect. What are the observations? How does the theory developed by Einstein explain them? 3. The particle nature of light can be explained using the concept of a photon. Remember the relationship between wavelength (wave property) and linear momentum (particle property). 4 ...
... 2. Photoelectric effect. What are the observations? How does the theory developed by Einstein explain them? 3. The particle nature of light can be explained using the concept of a photon. Remember the relationship between wavelength (wave property) and linear momentum (particle property). 4 ...
Lecture 2 EMS - San Jose State University
... • A beam of radiation (such as from the Sun) is usually polychromatic (has photons of different energies) • if only photons of one wavelength are involved the beam is monochromatic. • the distribution of all photon energies over the range of observed frequencies is embodied in the term spectrum ...
... • A beam of radiation (such as from the Sun) is usually polychromatic (has photons of different energies) • if only photons of one wavelength are involved the beam is monochromatic. • the distribution of all photon energies over the range of observed frequencies is embodied in the term spectrum ...
Do your homework on a separate piece of paper, or
... 35. List the four quantum numbers of the Schrödinger model. n, l, ml, and ms 36. Make an organized table of all of the allowed quantum states for energy level n = 3. Then label them s, p, and d. How many allowed states are there? See attachment. 37. What is the meaning of Pauli’s exclusion principle ...
... 35. List the four quantum numbers of the Schrödinger model. n, l, ml, and ms 36. Make an organized table of all of the allowed quantum states for energy level n = 3. Then label them s, p, and d. How many allowed states are there? See attachment. 37. What is the meaning of Pauli’s exclusion principle ...
Lecture 9 Introduction to Statistical Mechanics
... that light had both wave-like and particle-like behavior. Quantum mechanics is the result of reconciling classical mechanics with these microscopic observations. Instead of specifying a position and momentum, we must specify something that looks like a wave. The wave equation describes the probabili ...
... that light had both wave-like and particle-like behavior. Quantum mechanics is the result of reconciling classical mechanics with these microscopic observations. Instead of specifying a position and momentum, we must specify something that looks like a wave. The wave equation describes the probabili ...
III. Quantum Model of the Atom
... defines probability of finding an eTake it easy, do not get shocked, we will cover this in Chemy 333, if you are a chemistry major student ...
... defines probability of finding an eTake it easy, do not get shocked, we will cover this in Chemy 333, if you are a chemistry major student ...
Unit 3 Study Guide
... Explained optical spectra using the planetary Bohr Model of atom; First quantized model Uses orbits. Discovered neutrons Provided mathematical description of quantum mechanical orbitals Proposed the Uncertainty principle – the electron is only probably located in orbital Proposed electrons and other ...
... Explained optical spectra using the planetary Bohr Model of atom; First quantized model Uses orbits. Discovered neutrons Provided mathematical description of quantum mechanical orbitals Proposed the Uncertainty principle – the electron is only probably located in orbital Proposed electrons and other ...
Document
... quantum operators – The principle of complementarity • The Heisenberg uncertainty principle ...
... quantum operators – The principle of complementarity • The Heisenberg uncertainty principle ...
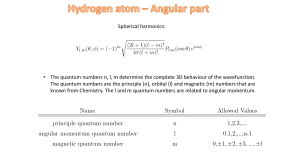
Spherical harmonics: • The quantum numbers n, l, m determine the
... result in nodal surfaces where the probability of finding the electron vanishes. This is because bound particles are standing waves! ...
... result in nodal surfaces where the probability of finding the electron vanishes. This is because bound particles are standing waves! ...
Solved Problems in the Quantum Theory of Light
... where we have written pe = h/λe . We eliminate φ and λe from these equations. Eliminating φ from last two equations yields ...
... where we have written pe = h/λe . We eliminate φ and λe from these equations. Eliminating φ from last two equations yields ...
Chapter 1 Introduction
... added by Einstein who showed in 1905 that the photoelectric effect could be explained by the hypothesis that the energy of a light beam was distributed in discrete packets later known as photons. Einstein also contributed to the understanding of the absorption and emission of light from atoms with h ...
... added by Einstein who showed in 1905 that the photoelectric effect could be explained by the hypothesis that the energy of a light beam was distributed in discrete packets later known as photons. Einstein also contributed to the understanding of the absorption and emission of light from atoms with h ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.