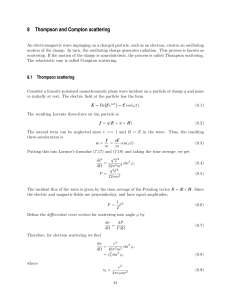
QUANTUM CHEMISTRY AND GROUP THEORY(2) M.Sc. DEGREE
... neither be derived nor be proved. These postulates provide a convenient framework for summarizing the basic concepts of quantum mechanics. The quantum mechanical postulates have been extensively tested since they were proposed. The predicted ...
... neither be derived nor be proved. These postulates provide a convenient framework for summarizing the basic concepts of quantum mechanics. The quantum mechanical postulates have been extensively tested since they were proposed. The predicted ...
- Snistnote
... numbers are required to specify completely each energy state. since for a particle inside the box, ‘ Ψ ’ cannot be zero, no quantum number can be zero. 2.The energy ‘ E ’ depends on the sum of the squares of the quantum numbers n1,n2 and n3 and no on their individual values. 3.Several combinations o ...
... numbers are required to specify completely each energy state. since for a particle inside the box, ‘ Ψ ’ cannot be zero, no quantum number can be zero. 2.The energy ‘ E ’ depends on the sum of the squares of the quantum numbers n1,n2 and n3 and no on their individual values. 3.Several combinations o ...
chapter 7 part 3
... state, this means eigen-value (energy) and wave function eigenfunction change let’s now consider how the particle returns to the ground state only if a transition form one wave function (m) to another wave function (n) is made, the energy changes ΔE = Em –En from one definitive value (excited statio ...
... state, this means eigen-value (energy) and wave function eigenfunction change let’s now consider how the particle returns to the ground state only if a transition form one wave function (m) to another wave function (n) is made, the energy changes ΔE = Em –En from one definitive value (excited statio ...
The Bohr model for the electrons
... The Quantum Mechanics: waves of uncertainty System developed that incorporated these concepts and produced an orbital picture of the electrons No longer think of electrons as particles with precise location, but as waves which have probability of being in some region of the atom – the orbital Impos ...
... The Quantum Mechanics: waves of uncertainty System developed that incorporated these concepts and produced an orbital picture of the electrons No longer think of electrons as particles with precise location, but as waves which have probability of being in some region of the atom – the orbital Impos ...
Paper : IIT-JEE Physics Question Paper Of Year 1999
... object P is kept at a distance of mR from it. Find the value of m for which a ray from P will emerge parallel to the table as shown in figure. (b) Photoelectrons are emitted when 400 nm radiation is incident on a surface of work function 1.9 eV. These photoelectrons pass through a region containing ...
... object P is kept at a distance of mR from it. Find the value of m for which a ray from P will emerge parallel to the table as shown in figure. (b) Photoelectrons are emitted when 400 nm radiation is incident on a surface of work function 1.9 eV. These photoelectrons pass through a region containing ...
physics 30 Matter assignment 4 - ND
... 14. The half-life of a theoretical radioactive isotope is 11.6 h. If there are 5.5 x 1022 atoms initially, how many atoms will remain after 48 h? a. ...
... 14. The half-life of a theoretical radioactive isotope is 11.6 h. If there are 5.5 x 1022 atoms initially, how many atoms will remain after 48 h? a. ...
Vocabulary:
... Nuclear Atom Model – An atom is mostly empty space with a dense, positively charged nucleus in the center and electrons moving around it. Neils Bohr – ...
... Nuclear Atom Model – An atom is mostly empty space with a dense, positively charged nucleus in the center and electrons moving around it. Neils Bohr – ...
Quantum Mechanics Booklet
... Capra states that science and mysticism can work hand-in-hand to discover the truth behind the universe. Similarly, McGrath states that science can help Christians to explain how Jesus can be both human and divine at the same time. Quantum mechanics reveals that there are some things that scie ...
... Capra states that science and mysticism can work hand-in-hand to discover the truth behind the universe. Similarly, McGrath states that science can help Christians to explain how Jesus can be both human and divine at the same time. Quantum mechanics reveals that there are some things that scie ...
Chapter 6 Outline full
... Electron density is another way of expressing probability. • A region of high electron density is one where there is a high probability of finding an electron. ...
... Electron density is another way of expressing probability. • A region of high electron density is one where there is a high probability of finding an electron. ...
Physics 125a – Problem Set 5 – Due Nov 12,... Version 3 – Nov 11, 2007
... Section 5. Finally, some real quantum mechanics! v. 2: Provide result for transmission as a function of wavevector in (5b). More specificity on how to do plot. v. 3: In (5b), had mistakenly written k1 and k2 as if the well were at −V0 and the potential was zero elsewhere, instead of what is given, w ...
... Section 5. Finally, some real quantum mechanics! v. 2: Provide result for transmission as a function of wavevector in (5b). More specificity on how to do plot. v. 3: In (5b), had mistakenly written k1 and k2 as if the well were at −V0 and the potential was zero elsewhere, instead of what is given, w ...
URL - StealthSkater
... object acts as a single "giant electron" if -- and only if -- the shape of the object represents the actual physical shape of a relativistic electron. By "relativistic electron", I mean an electron that’s traveling at very close to the speed-of-light will have a certain shape. So if we want to behav ...
... object acts as a single "giant electron" if -- and only if -- the shape of the object represents the actual physical shape of a relativistic electron. By "relativistic electron", I mean an electron that’s traveling at very close to the speed-of-light will have a certain shape. So if we want to behav ...
The hydrogen line spectrum explained as Raman shift
... energy difference of only 0.000045 eV because energy is allegedly E = hν. If this were the case, the relative intensity difference of 50% for the two red lines is not explicable. Remember that in terms of quantum theory the intensity of a line means the number of photon shots and that all photons as ...
... energy difference of only 0.000045 eV because energy is allegedly E = hν. If this were the case, the relative intensity difference of 50% for the two red lines is not explicable. Remember that in terms of quantum theory the intensity of a line means the number of photon shots and that all photons as ...
A logico-conceptual analysis of the Einstein-Podolsky
... Step 1 follows immediately from the completeness condition. Steps 3, 4, 6, 7 and 8 are a matter of elementary logic. Step 2 is a direct consequence of quantum mechanical formalism; but curiously EPR extend its discussion. First, they show explicitly that QMAB is false when A and B are the position a ...
... Step 1 follows immediately from the completeness condition. Steps 3, 4, 6, 7 and 8 are a matter of elementary logic. Step 2 is a direct consequence of quantum mechanical formalism; but curiously EPR extend its discussion. First, they show explicitly that QMAB is false when A and B are the position a ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.