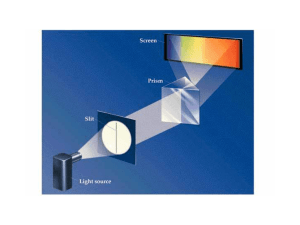
Introduction
... • Since the interference pattern disappears when one of the slits is covered, why then this phenomena changes so drastically? • Crucial: the process of measurement • When one performs a measurement on a microscopic system, one disturbs it in a fundamental fashion • It is impossible to observe the in ...
... • Since the interference pattern disappears when one of the slits is covered, why then this phenomena changes so drastically? • Crucial: the process of measurement • When one performs a measurement on a microscopic system, one disturbs it in a fundamental fashion • It is impossible to observe the in ...
19.1 Reinforcement WKT to project
... 1. How is the chemical symbol of an element determined? 2. Of what are atoms composed? 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? 4. How many types of quarks are there and what is the name of one of them? 5. Why do scientists use models to study atoms? 6. Why ha ...
... 1. How is the chemical symbol of an element determined? 2. Of what are atoms composed? 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? 4. How many types of quarks are there and what is the name of one of them? 5. Why do scientists use models to study atoms? 6. Why ha ...
Academic Chemistry Atomic History Study Guide 1. Identify and
... eventually lead to the production of nuclear weapons, provided insight into the internal structure and composition of the atomic nucleus. Describe their discovery. 16. _______________ ____________________ developed mathematical equations which allowed super computers to calculate the probability of ...
... eventually lead to the production of nuclear weapons, provided insight into the internal structure and composition of the atomic nucleus. Describe their discovery. 16. _______________ ____________________ developed mathematical equations which allowed super computers to calculate the probability of ...
File
... quantum mechanics earned him the 1929 Nobel Prize in Physics. His doctoral thesis, which proposed that all particles have a characteristic wavelength dependent on their momentum, was so groundbreaking that the reviewers passed it directly to Einstein, who endorsed it. In opposition to the probabilis ...
... quantum mechanics earned him the 1929 Nobel Prize in Physics. His doctoral thesis, which proposed that all particles have a characteristic wavelength dependent on their momentum, was so groundbreaking that the reviewers passed it directly to Einstein, who endorsed it. In opposition to the probabilis ...
Chapter 8 - Fayetteville State University
... 1) Photoelectric effect: emission of electrons from a metallic surface when light shines on it. 1) Photons: Small packets of energy from which light is made up and through which light propagates. 2) X-rays: are high-frequency electromagnetic radiation given off when matter is struck by fast electron ...
... 1) Photoelectric effect: emission of electrons from a metallic surface when light shines on it. 1) Photons: Small packets of energy from which light is made up and through which light propagates. 2) X-rays: are high-frequency electromagnetic radiation given off when matter is struck by fast electron ...
Thesis Presentation Mr. Joshuah T. Heath Department of Physics
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
No Slide Title
... the electron is described by a wave function, Y. The exact wavefunction for each electron depends upon four variables, called quantum numbers they are Erwin Schrodinger, an Austrian physicist, proposed that we think of the electrons more as waves than particles. This led to the field called quantum ...
... the electron is described by a wave function, Y. The exact wavefunction for each electron depends upon four variables, called quantum numbers they are Erwin Schrodinger, an Austrian physicist, proposed that we think of the electrons more as waves than particles. This led to the field called quantum ...
Quantum_PPT
... older wave theory: • 1. Wave theory could not explain why a hot body emitted light. • 2. UV-light discharged electricallycharged metal plates (the photoelectric effect). ...
... older wave theory: • 1. Wave theory could not explain why a hot body emitted light. • 2. UV-light discharged electricallycharged metal plates (the photoelectric effect). ...
An introduction to Quantum Optics
... 1) Problem of interpretation 2) Problem of formalism : many diverging quantities e.g. Vacuum energy : 3) Problem of "concurrence" : the more simple semiclassical theory gives (generally) the same results • 2) was solved in 1947 (Feynman, Schwinger & Tomonaga) : ...
... 1) Problem of interpretation 2) Problem of formalism : many diverging quantities e.g. Vacuum energy : 3) Problem of "concurrence" : the more simple semiclassical theory gives (generally) the same results • 2) was solved in 1947 (Feynman, Schwinger & Tomonaga) : ...
document
... Obtain the positions of the bright and dark fringes measured vertically from O to P Assume that L >> d and d >> L = the order of 1 m, d = a fraction of a millimeter, and = a fraction of a micrometer for visible light ...
... Obtain the positions of the bright and dark fringes measured vertically from O to P Assume that L >> d and d >> L = the order of 1 m, d = a fraction of a millimeter, and = a fraction of a micrometer for visible light ...
Document
... A thought experiment for viewing an electron with a powerful microscope In order to see the electron, at least one photon must bounce off it During this interaction, momentum is transferred from the photon to the electron Therefore, the light that allows you to accurately locate the electron changes ...
... A thought experiment for viewing an electron with a powerful microscope In order to see the electron, at least one photon must bounce off it During this interaction, momentum is transferred from the photon to the electron Therefore, the light that allows you to accurately locate the electron changes ...
Lecture Notes, Feb 29
... The idea of the position of an object seems so obvious that the concept of position is generally taken for granted in classical physics. Knowing the position of a particle means knowing the values of its coordinates in some coordinate system. The precision of those values, in classical physics, is l ...
... The idea of the position of an object seems so obvious that the concept of position is generally taken for granted in classical physics. Knowing the position of a particle means knowing the values of its coordinates in some coordinate system. The precision of those values, in classical physics, is l ...
Overview of Particle Physics
... large energy is not sufficient to reveal the nucleon constituents! At large beam particle energy the target does not break up into constituents! new particles are created! mass is not conserved! ...
... large energy is not sufficient to reveal the nucleon constituents! At large beam particle energy the target does not break up into constituents! new particles are created! mass is not conserved! ...
Chapter 24
... Two rectangular optically flat plates (n = 1.52) are in contact along one end and are separated along the other end by a 2.00-mm- thick spacer. The top plate is illuminated by monochromatic light of wavelength 546.1 nm. Calculate the number of dark parallel bands crossing the top plate (including th ...
... Two rectangular optically flat plates (n = 1.52) are in contact along one end and are separated along the other end by a 2.00-mm- thick spacer. The top plate is illuminated by monochromatic light of wavelength 546.1 nm. Calculate the number of dark parallel bands crossing the top plate (including th ...