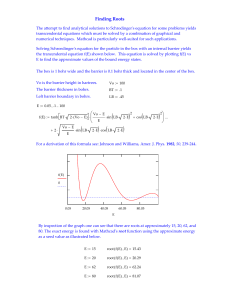
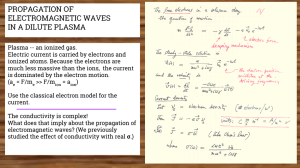
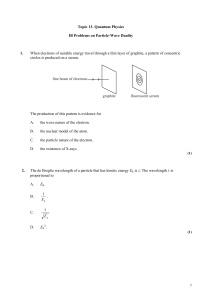
The world of Atoms - University of California, Irvine
... Theory that describes the physical properties of smallest particles (atoms, protons, electrons, photons) Max Planck "A scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die and a new generation grows up that is fa ...
... Theory that describes the physical properties of smallest particles (atoms, protons, electrons, photons) Max Planck "A scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die and a new generation grows up that is fa ...
Definitions are in Book
... change in enthalpy for whole process is the same, regardless of whether you use Hess’s law or heats of formation. In this way enthalpy is a state function. 3) What is meant by “Energy is quantized”? Energy can only be certain values. For example, the 1s orbital has a certain energy and the 2s orbita ...
... change in enthalpy for whole process is the same, regardless of whether you use Hess’s law or heats of formation. In this way enthalpy is a state function. 3) What is meant by “Energy is quantized”? Energy can only be certain values. For example, the 1s orbital has a certain energy and the 2s orbita ...
Quantum physics
... • the maximum kinetic energy increases linearly with frequency •the electrons are emitted almost instantaneously, even at very low light intensities These observations contradict the classical description. It suggest that energy is delivered to the electrons in the metal in terms of localized packet ...
... • the maximum kinetic energy increases linearly with frequency •the electrons are emitted almost instantaneously, even at very low light intensities These observations contradict the classical description. It suggest that energy is delivered to the electrons in the metal in terms of localized packet ...
Chapter 5
... Photons are radiation with no mass that carries a quantum of energy. Einstein calculated that a photon’s energy depends on its frequency: Ephoton = h ...
... Photons are radiation with no mass that carries a quantum of energy. Einstein calculated that a photon’s energy depends on its frequency: Ephoton = h ...
Wave Particle Duality - waiukucollegescience
... Acceleration due to gravity,g = 9.81 Nkg-1 Speed of light = 3.0 x 108ms-1 Planck's constant = 6.6 x 10-34Js Mass of electron = 9.1 x 10-31kg Electronic charge = 1.6 x 10-19C (1) When light is incident in a metal plate electrons are emitted only when the frequency of the light exceeds a certain value ...
... Acceleration due to gravity,g = 9.81 Nkg-1 Speed of light = 3.0 x 108ms-1 Planck's constant = 6.6 x 10-34Js Mass of electron = 9.1 x 10-31kg Electronic charge = 1.6 x 10-19C (1) When light is incident in a metal plate electrons are emitted only when the frequency of the light exceeds a certain value ...
Wave-particle_duality
... An α-particle having a de Broglie wavelength λi collides with a stationary carbon nucleus. The α-particle moves off in a different direction as shown below. final direction of –particle, de Broglie wavelength f ...
... An α-particle having a de Broglie wavelength λi collides with a stationary carbon nucleus. The α-particle moves off in a different direction as shown below. final direction of –particle, de Broglie wavelength f ...
Periodic Boundary Conditions. Classical Limit ( + problems 27
... energy is of order T . That is it is just a typical de Broglie wavelength corresponding to a given temperature at a given particle mass. Now we see that the inequality (19) is the requirement that typical de Broglie wavelength be much smaller than the system size: λT ¿ L . ...
... energy is of order T . That is it is just a typical de Broglie wavelength corresponding to a given temperature at a given particle mass. Now we see that the inequality (19) is the requirement that typical de Broglie wavelength be much smaller than the system size: λT ¿ L . ...
Slide 1
... 2. Photoelectric Effect - light shining on a clean metal surface causes the surface to emit electrons if the light is of a certain minimum frequency . http://web2.uwindsor.ca/courses/physics/high_schools/2005/Photoelectric_effect/hist.html 3. He said light energy hitting a metal surface is not like ...
... 2. Photoelectric Effect - light shining on a clean metal surface causes the surface to emit electrons if the light is of a certain minimum frequency . http://web2.uwindsor.ca/courses/physics/high_schools/2005/Photoelectric_effect/hist.html 3. He said light energy hitting a metal surface is not like ...