Formula Sheet File - Eastchester High School
... (If not at top or bottom of circle, you might have to find components of Fg (if FT is toward center) or find components of FT (If FT is NOT toward center)) ...
... (If not at top or bottom of circle, you might have to find components of Fg (if FT is toward center) or find components of FT (If FT is NOT toward center)) ...
Quantum Tunneling - Santa Rosa Junior College
... energy, time, momentum…) can only be described as distributions of probability. These distributions have another limitation. Due to our methods of detection, we are restricted to never knowing two properties of a particle simultaneously. We can never understand fully (with our current knowledge) wha ...
... energy, time, momentum…) can only be described as distributions of probability. These distributions have another limitation. Due to our methods of detection, we are restricted to never knowing two properties of a particle simultaneously. We can never understand fully (with our current knowledge) wha ...
Lecture 8 1 Schrodinger equation (continued)
... Let’s assume that atoms are very tiny (≈ 10−10 meter) 1-D boxes with very hard walls. The walls are located at position x = 0 and x = l. This model works surprisingly well. Inside the box Ĥ is given by the h̄2 ∂ 2 free particle Hamiltonian Ĥ = − 2m . Outside the box we model the very hard walls as ...
... Let’s assume that atoms are very tiny (≈ 10−10 meter) 1-D boxes with very hard walls. The walls are located at position x = 0 and x = l. This model works surprisingly well. Inside the box Ĥ is given by the h̄2 ∂ 2 free particle Hamiltonian Ĥ = − 2m . Outside the box we model the very hard walls as ...
Introduction to quantum mechanics
... The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom. The only information that was important was the size of the orbit, which was described by the n quantum number. Schrödinger's model allowed the electron to occupy three-dime ...
... The Bohr model was a one-dimensional model that used one quantum number to describe the distribution of electrons in the atom. The only information that was important was the size of the orbit, which was described by the n quantum number. Schrödinger's model allowed the electron to occupy three-dime ...
Ch 5 - Electrons in Atoms
... Einstein used this theory to explain why metals (and some semi-metals) will eject e- from the surface when light of specific a frequency hits it. • This is called the photoelectric effect • Used with solar panels ...
... Einstein used this theory to explain why metals (and some semi-metals) will eject e- from the surface when light of specific a frequency hits it. • This is called the photoelectric effect • Used with solar panels ...
Physics 161 NAME
... It is meaningless to compare the amount of work because the forces were so different. e. Work was done on B, but no work was done on A because the wall did not move. ...
... It is meaningless to compare the amount of work because the forces were so different. e. Work was done on B, but no work was done on A because the wall did not move. ...
CE2
... A train of straight water waves travels towards a straight barrier with two openings. Two sets of circular waves are produced on the other side of the barrier. The distance of a point P from S1 and S2 are 35 cm and 30 cm respectively. Suppose constructive interference occurs at P. What is the possib ...
... A train of straight water waves travels towards a straight barrier with two openings. Two sets of circular waves are produced on the other side of the barrier. The distance of a point P from S1 and S2 are 35 cm and 30 cm respectively. Suppose constructive interference occurs at P. What is the possib ...
Starter
... with a force of 10 N? 4. It takes 8 seconds for a pulley system to lift a load with 400 N.m. How much power is required? ...
... with a force of 10 N? 4. It takes 8 seconds for a pulley system to lift a load with 400 N.m. How much power is required? ...
A New Principle of Conservation of Energy
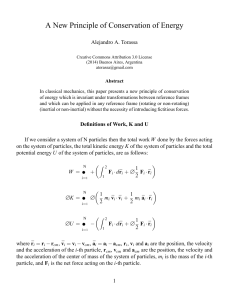
... Theorems of K and U In a system of N particles, the total work W done by the forces acting on the system of particles is equal to the change in the total kinetic energy K of the system of particles. W = +∆ K In a system of N particles, the total work W done by the conservative forces acting on the ...
... Theorems of K and U In a system of N particles, the total work W done by the forces acting on the system of particles is equal to the change in the total kinetic energy K of the system of particles. W = +∆ K In a system of N particles, the total work W done by the conservative forces acting on the ...