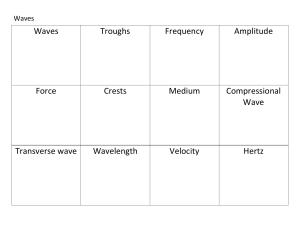
2 - web.pdx.edu
... wave, but wait a minute – we just get the same result for electrons – that’s strange ---------------------------------------------------------------so is light kind of a wave that consist of particles, or is it kind of particles that are guided/piloted by a wave ? light as an electromagnetic wave: e ...
... wave, but wait a minute – we just get the same result for electrons – that’s strange ---------------------------------------------------------------so is light kind of a wave that consist of particles, or is it kind of particles that are guided/piloted by a wave ? light as an electromagnetic wave: e ...
E489: Decay of a particle with spin 0
... (3) I’m afraid the answer still eludes me. I have discussed the question with DC, but after further thought I came to disagree with his point of view, which I will lay out here, to promote discussion on the matter. In order for measurement to be taken, the particles have to reach the detectors, so w ...
... (3) I’m afraid the answer still eludes me. I have discussed the question with DC, but after further thought I came to disagree with his point of view, which I will lay out here, to promote discussion on the matter. In order for measurement to be taken, the particles have to reach the detectors, so w ...
1 ψ ω ω ω ψ ψ ψ
... for 0 ≤ x ≤ L and zero otherwise. (a) Determine the expectation value of x. (b) Determine the probability of finding the particle near L/2, by calculating the probability that the particle lies in the range 0.490L ≤ x ≤ 0.510L. (c) What If? Determine the probability of finding the particle near L/4, ...
... for 0 ≤ x ≤ L and zero otherwise. (a) Determine the expectation value of x. (b) Determine the probability of finding the particle near L/2, by calculating the probability that the particle lies in the range 0.490L ≤ x ≤ 0.510L. (c) What If? Determine the probability of finding the particle near L/4, ...
Introduction to Quantum Mechanic
... of an electron exactly. Rather, it provides only a probability as to where the electron will be found. We shall illustrate the probability aspect in terms of the system of an electron confined to motion along a line of length L. Quantum mechanical probabilities are expressed in terms of a distributi ...
... of an electron exactly. Rather, it provides only a probability as to where the electron will be found. We shall illustrate the probability aspect in terms of the system of an electron confined to motion along a line of length L. Quantum mechanical probabilities are expressed in terms of a distributi ...
Slide 1
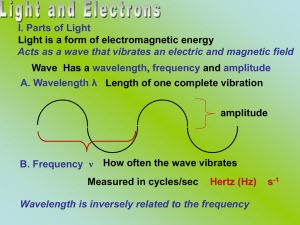
... II. Light as a Particle A. Max Planck Light is released in small units quanta Energy of these units is related to frequency of light E = energy of quantum (J) E = hv v = frequency (s-1) h = Planck’s constant (6.63 x 10-34 J-s) What is the energy of a photon with a wavelength of 236 nm? ...
... II. Light as a Particle A. Max Planck Light is released in small units quanta Energy of these units is related to frequency of light E = energy of quantum (J) E = hv v = frequency (s-1) h = Planck’s constant (6.63 x 10-34 J-s) What is the energy of a photon with a wavelength of 236 nm? ...
Chapter 3 Wave Properties of Particles Overview
... in the case of X-rays one had both waves and corpuscles, thus suddenly - ... it was certain in the course of summer 1923 - I got the idea that one had to extend this duality to material particles, especially to electrons. And I realised that, on the one hand, the Hamilton-Jacobi theory pointed somew ...
... in the case of X-rays one had both waves and corpuscles, thus suddenly - ... it was certain in the course of summer 1923 - I got the idea that one had to extend this duality to material particles, especially to electrons. And I realised that, on the one hand, the Hamilton-Jacobi theory pointed somew ...
THINGSYOUNEEDTOKNOW-modern
... CONTINUOUS SPECTRUM-A smooth spectrum of all colors of the rainbow. Generated by light bulb ABSORPTION SPECTRUM-looks like continuous spectrum with some spectral lines absorbed By a cool gas EMISSION SPECTRUM-a spectrum made up of only a few spectral lines emitted by an excited gas. Energy of photon ...
... CONTINUOUS SPECTRUM-A smooth spectrum of all colors of the rainbow. Generated by light bulb ABSORPTION SPECTRUM-looks like continuous spectrum with some spectral lines absorbed By a cool gas EMISSION SPECTRUM-a spectrum made up of only a few spectral lines emitted by an excited gas. Energy of photon ...
Precursors to Modern Physics
... Why the energy state ordering of an electron in an atom is affected by large orbital quantum numbers? The state of an electron in an atom is completely defined by its quantum numbers. The energy of the electron is also a function of Z, the total positive charge of the nucleus. For the electrons with ...
... Why the energy state ordering of an electron in an atom is affected by large orbital quantum numbers? The state of an electron in an atom is completely defined by its quantum numbers. The energy of the electron is also a function of Z, the total positive charge of the nucleus. For the electrons with ...
Topic 11 — relativity - energy and momentum — Use the
... bundled into something that satisfies this for some particular real m (so that m2 ≥ 0) that is characteristic of the particle. All electrons have the mass of the electron. All protons have the mass of a proton. Etc. The nice thing about this relation is that it makes sense even if m = 0. This is imp ...
... bundled into something that satisfies this for some particular real m (so that m2 ≥ 0) that is characteristic of the particle. All electrons have the mass of the electron. All protons have the mass of a proton. Etc. The nice thing about this relation is that it makes sense even if m = 0. This is imp ...
Lecture 25: Wave mechanics
... Thus the electron wavelength is comparable to typical molecular bond length! To describe the motion as well as location of this electron wave we must develop an equation like Newton’s force =ma equation. How can we use Bohr’s picture to define the electron as a wave in it’s own orbit? ...
... Thus the electron wavelength is comparable to typical molecular bond length! To describe the motion as well as location of this electron wave we must develop an equation like Newton’s force =ma equation. How can we use Bohr’s picture to define the electron as a wave in it’s own orbit? ...
Energy
... • A “particle” of light • A “quantum” of light energy • The energy of a given photon depends on the frequency (color) of the light ...
... • A “particle” of light • A “quantum” of light energy • The energy of a given photon depends on the frequency (color) of the light ...
Lecture 2
... Figure 1.8: The classical light experiment that showed that light behaves like waves because of the observed interference patterns when two circular waves that emerge from closely spaced slits emerge. When one slit is blocked, the interference pattern disappears. a source emits light, the light hit ...
... Figure 1.8: The classical light experiment that showed that light behaves like waves because of the observed interference patterns when two circular waves that emerge from closely spaced slits emerge. When one slit is blocked, the interference pattern disappears. a source emits light, the light hit ...
FIZICA
... S10. The Pauli principle states that: Into an atom (molecule) there are no two electrons (in general fermions) characterized by identical quantum numbers. Name these four quantum numbers and calculate the maximum number of orbitals for a hidrogenoid atom with two electrons characterized by i) n1 = 2 ...
... S10. The Pauli principle states that: Into an atom (molecule) there are no two electrons (in general fermions) characterized by identical quantum numbers. Name these four quantum numbers and calculate the maximum number of orbitals for a hidrogenoid atom with two electrons characterized by i) n1 = 2 ...
Quantum Physics - The University of Sydney
... This module is one of three comprising PHYS 1902 Physics 1 (Advanced). This document describes details of this module and should be read in conjunction with the more general unit of study outline for PHYS 1902 Physics 1 (Advanced). ...
... This module is one of three comprising PHYS 1902 Physics 1 (Advanced). This document describes details of this module and should be read in conjunction with the more general unit of study outline for PHYS 1902 Physics 1 (Advanced). ...
Document
... • E = hf, the quantum of energy for light. (PE effect & black body rad.) • f = c/l, c = 3E8m/sec, l = wavelength • From Poynting’s theorem (em waves), momentum density = energy density/c • Postulate a Photon “momentum” p = h/l = hk, h = h/2p wavenumber, k = 2p /l L1 January 18 ...
... • E = hf, the quantum of energy for light. (PE effect & black body rad.) • f = c/l, c = 3E8m/sec, l = wavelength • From Poynting’s theorem (em waves), momentum density = energy density/c • Postulate a Photon “momentum” p = h/l = hk, h = h/2p wavenumber, k = 2p /l L1 January 18 ...