Statistics/Mathematics 309 Larget September 16, 2009 Assignment #3
... 1. A fair coin is tossed 15 times. What is the probability that the length of the longest run of heads is exactly 5? Hint: follow the example from lecture using a recursion relation. 2. A coin is tossed four times. Assume that the coin tosses are independent and that heads and tails are equally like ...
... 1. A fair coin is tossed 15 times. What is the probability that the length of the longest run of heads is exactly 5? Hint: follow the example from lecture using a recursion relation. 2. A coin is tossed four times. Assume that the coin tosses are independent and that heads and tails are equally like ...
WAVE MECHANICS (Schrödinger, 1926)
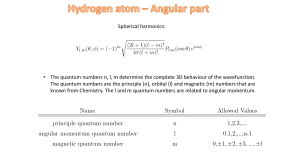
... * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All orbitals with the same n and l are called a “subshell”. ...
... * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All orbitals with the same n and l are called a “subshell”. ...
Modern Physics
... There is a fundamental limit to the accuracy of a measurement determined by the Heisenberg uncertainty principle If a measurement of position is made with precision Dx and a simultaneous measurement of linear momentum is made with precision Dp, then the product of the two uncertainties can never be ...
... There is a fundamental limit to the accuracy of a measurement determined by the Heisenberg uncertainty principle If a measurement of position is made with precision Dx and a simultaneous measurement of linear momentum is made with precision Dp, then the product of the two uncertainties can never be ...
from last time:
... often done by educated guessing, and there may be more than one solution. Apply boundary conditions – these will often limit your values of energy. Evaluate any undetermined constants (like amplitudes), e.g. by using boundary conditions, applying normalisation. Check your solution, if it gives you s ...
... often done by educated guessing, and there may be more than one solution. Apply boundary conditions – these will often limit your values of energy. Evaluate any undetermined constants (like amplitudes), e.g. by using boundary conditions, applying normalisation. Check your solution, if it gives you s ...
Negative Binomial Distribution
... Tail Probability Example: If 30% of the cars passing your house are red what is the probability that more than 5 cars will pass before you observe the first red one? (Geometric Distribution: a special case of Negative Binomial Distribution.) 1.) In R Commander, select IPSUR-Probability, Discrete Dis ...
... Tail Probability Example: If 30% of the cars passing your house are red what is the probability that more than 5 cars will pass before you observe the first red one? (Geometric Distribution: a special case of Negative Binomial Distribution.) 1.) In R Commander, select IPSUR-Probability, Discrete Dis ...
Document
... f (x, y) and marginal X pdf fX(x). Then for any X value x for which fX(x) > 0, the conditional probability density function of Y given that X = x is If X and Y are discrete, replacing pdf’s by pmf’s gives the conditional probability mass function of Y when X = x. ...
... f (x, y) and marginal X pdf fX(x). Then for any X value x for which fX(x) > 0, the conditional probability density function of Y given that X = x is If X and Y are discrete, replacing pdf’s by pmf’s gives the conditional probability mass function of Y when X = x. ...
Introduction to Electromagnetism
... Light interferes and diffracts - but so do electrons! in Ni crystal ...
... Light interferes and diffracts - but so do electrons! in Ni crystal ...
lecture
... ■ Calculate probability of events under assumptions of regularity and consistency (theoretically or empirically determined) ■ Determine how likely our event is, and then interpret it accordingly. ...
... ■ Calculate probability of events under assumptions of regularity and consistency (theoretically or empirically determined) ■ Determine how likely our event is, and then interpret it accordingly. ...
Statistical laws
... §1 Statistical laws of macroscopic matter Statistical laws In classic physics, the motion of a single particle will obey Newton’s law. If the initial position and velocity are known, we can predict its position at any time by solving the Newton equation of motions. A macroscopic body has a large ...
... §1 Statistical laws of macroscopic matter Statistical laws In classic physics, the motion of a single particle will obey Newton’s law. If the initial position and velocity are known, we can predict its position at any time by solving the Newton equation of motions. A macroscopic body has a large ...
WHY STUDY QUANTUM CHEMISTRY? Physical Chemisty can be
... changes with time. Since it explains physical phenomena, the postulate must be correct. For an n-particle system: -(h/i)(∂Ψ/ ∂t) = -(h2/2m1)(∂2Ψ/∂x12+∂2 Ψ/∂y12+∂2Ψ/∂z12) + ... -(h2/2mn)(∂2Ψ/∂xn2+∂2 Ψ/∂yn2+∂2Ψ/∂zn2) + V Ψ where mi is the mass, (xi, yi, zi) are the Cartesian coordinates of particle i, ...
... changes with time. Since it explains physical phenomena, the postulate must be correct. For an n-particle system: -(h/i)(∂Ψ/ ∂t) = -(h2/2m1)(∂2Ψ/∂x12+∂2 Ψ/∂y12+∂2Ψ/∂z12) + ... -(h2/2mn)(∂2Ψ/∂xn2+∂2 Ψ/∂yn2+∂2Ψ/∂zn2) + V Ψ where mi is the mass, (xi, yi, zi) are the Cartesian coordinates of particle i, ...
Quantum Theory 1 - Home Exercise 9
... (c) A measurement of L̂2 yields 6~2 . Afterwards, we measure L̂z . What are the possible values in this measurement? What is the probabilities of measuring any of these values? (d) What is the probability to measure L̂x = 0? ...
... (c) A measurement of L̂2 yields 6~2 . Afterwards, we measure L̂z . What are the possible values in this measurement? What is the probabilities of measuring any of these values? (d) What is the probability to measure L̂x = 0? ...
Probability amplitude

In quantum mechanics, a probability amplitude is a complex number used in describing the behaviour of systems. The modulus squared of this quantity represents a probability or probability density.Probability amplitudes provide a relationship between the wave function (or, more generally, of a quantum state vector) of a system and the results of observations of that system, a link first proposed by Max Born. Interpretation of values of a wave function as the probability amplitude is a pillar of the Copenhagen interpretation of quantum mechanics. In fact, the properties of the space of wave functions were being used to make physical predictions (such as emissions from atoms being at certain discrete energies) before any physical interpretation of a particular function was offered. Born was awarded half of the 1954 Nobel Prize in Physics for this understanding (see #References), and the probability thus calculated is sometimes called the ""Born probability"". These probabilistic concepts, namely the probability density and quantum measurements, were vigorously contested at the time by the original physicists working on the theory, such as Schrödinger and Einstein. It is the source of the mysterious consequences and philosophical difficulties in the interpretations of quantum mechanics—topics that continue to be debated even today.