PREPARATIONS AND APPLICATION OF METAL NANOPARTICLES
... Pd nanocluster stabilized by tetraalkylammonium ions. Tetraalkylammonium ions in the solvent of CH3CN/THF functioned as a supporting electrolyte and a stabilizer. Sacrificial electrode was used as a metal source. It is electrolyzed to release metal ions and are subsequently reduced at cathode. The m ...
... Pd nanocluster stabilized by tetraalkylammonium ions. Tetraalkylammonium ions in the solvent of CH3CN/THF functioned as a supporting electrolyte and a stabilizer. Sacrificial electrode was used as a metal source. It is electrolyzed to release metal ions and are subsequently reduced at cathode. The m ...
Synthesis, Crystal-Structure Determination and Magnetic Properties
... deduced by a comparison with the crystal structure of CuNCN and earlier quantum-chemical predictions.2,3 The backgrounds of the data set were manually subtracted by linear interpolation, and the FULLPROF program package6 was used for the Rietveld refinements using a pseudo-Voigt profile function, re ...
... deduced by a comparison with the crystal structure of CuNCN and earlier quantum-chemical predictions.2,3 The backgrounds of the data set were manually subtracted by linear interpolation, and the FULLPROF program package6 was used for the Rietveld refinements using a pseudo-Voigt profile function, re ...
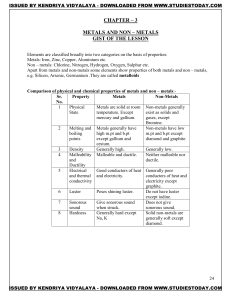
METALS AND NON – METALS Concepts
... Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, Germanium .They are called metalloids Comparison of physical and chemical ...
... Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, Germanium .They are called metalloids Comparison of physical and chemical ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood (Pages 735
... *2. INSOLUBLE SOLUTES that will NOT dissolve in a SOLVENT *3. SATURATED are solutions with so much SOLUTE that no more will DISSOLVE *4. UNSATURATED are solutions where MORE solute will continue to DISSOLVE when added to the SOLVENT *5. SUPERSATURATED are solutions containing more SOLUTE tha ...
... *2. INSOLUBLE SOLUTES that will NOT dissolve in a SOLVENT *3. SATURATED are solutions with so much SOLUTE that no more will DISSOLVE *4. UNSATURATED are solutions where MORE solute will continue to DISSOLVE when added to the SOLVENT *5. SUPERSATURATED are solutions containing more SOLUTE tha ...
Summary notes - Kelso High School
... Acids and pH The word ACID means sour. We often say that a sour liquid tastes acid. Examples of sour liquids that you will find in the home are vinegar, lemon juice, grapefruit juice and sour milk. You can find out if a substance is an acid or and alkali by dissolving it in water and adding an indi ...
... Acids and pH The word ACID means sour. We often say that a sour liquid tastes acid. Examples of sour liquids that you will find in the home are vinegar, lemon juice, grapefruit juice and sour milk. You can find out if a substance is an acid or and alkali by dissolving it in water and adding an indi ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... 2. Mixtures are not ______ substances (not an element or compound) 3. Components of a MIXTURE are NOT all IDENTICAL and do NOT have DEFINITE properties because they do NOT have a defined __________________ makeup 4. Mixtures can be __________________ by PHYSICAL means which depends on their physical ...
... 2. Mixtures are not ______ substances (not an element or compound) 3. Components of a MIXTURE are NOT all IDENTICAL and do NOT have DEFINITE properties because they do NOT have a defined __________________ makeup 4. Mixtures can be __________________ by PHYSICAL means which depends on their physical ...
Unit 2.7: Periodic Table Group1 Group2 Li Be Na Mg K Ca Rb Sr Cs
... because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements in the same period. The delocalized electrons in the metal are mobile. Therefore they can move ...
... because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements in the same period. The delocalized electrons in the metal are mobile. Therefore they can move ...
SAMPLE QUESTION PAPER CHEMISTRY (043) CLASS XII (2013-14)
... 27. (a) Artificial sweetening agents that are non-nutritive in nature are used as substituents for sugar (specially in soft drinks). Examples are saccharin (500 times sweeter than sucrose) and ...
... 27. (a) Artificial sweetening agents that are non-nutritive in nature are used as substituents for sugar (specially in soft drinks). Examples are saccharin (500 times sweeter than sucrose) and ...
Chemical Synthesis Using Earth-Abundant Metal
... (i.e., Pd, Pt, Ru, Rh, Ir, Ag and Au). The problem with precious metals is that they are expensive, steadily rarefying, and are generally non-renewable. Catalysts made from these metals can also be harmful to humans and to the environment. ...
... (i.e., Pd, Pt, Ru, Rh, Ir, Ag and Au). The problem with precious metals is that they are expensive, steadily rarefying, and are generally non-renewable. Catalysts made from these metals can also be harmful to humans and to the environment. ...
Mixture Solution Notes
... 1. What does “dissolve” mean? 2. What kinds of things dissolve? 3. What do things dissolve in? ...
... 1. What does “dissolve” mean? 2. What kinds of things dissolve? 3. What do things dissolve in? ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... 2. Mixtures are not ______ substances (not an element or compound) 3. Components of a MIXTURE are NOT all IDENTICAL and do NOT have DEFINITE properties because they do NOT have a defined __________________ makeup 4. Mixtures can be __________________ by PHYSICAL means which depends on their physical ...
... 2. Mixtures are not ______ substances (not an element or compound) 3. Components of a MIXTURE are NOT all IDENTICAL and do NOT have DEFINITE properties because they do NOT have a defined __________________ makeup 4. Mixtures can be __________________ by PHYSICAL means which depends on their physical ...
Activity series
... Group Roles: A Technician; B Leader; C Recorder Redox reactions are some of the most common and most useful chemical reactions. They produce electrical current which can be harnessed to do work. Transition metals play a very important role in redox chemistry. Questions: Which metals are easily oxidi ...
... Group Roles: A Technician; B Leader; C Recorder Redox reactions are some of the most common and most useful chemical reactions. They produce electrical current which can be harnessed to do work. Transition metals play a very important role in redox chemistry. Questions: Which metals are easily oxidi ...
Practice Problems
... • Making potassium nitride from its component elements • Uranium (VI) fluoride reacts with magnesium metal ...
... • Making potassium nitride from its component elements • Uranium (VI) fluoride reacts with magnesium metal ...
activity series
... occurs between ions in aqueous solution. A reaction will occur when a pair of ions come together to produce at least one of the following: 1. a precipitate 2. a gas 3. water or some other non-ionized substance. ...
... occurs between ions in aqueous solution. A reaction will occur when a pair of ions come together to produce at least one of the following: 1. a precipitate 2. a gas 3. water or some other non-ionized substance. ...
CERAMICS MATERIALS - Wits Structural Chemistry
... The 3d-metal oxides such as MnO, FeO, CoO and NiO are semiconductors and TiO and VO are metallic conductors. 3d-metal oxides - MnO, Fe1-xO, CoO and NiO have low conductivity that increase with temperature or have such large band gaps that become insulators. The electron-hole migration in these oxide ...
... The 3d-metal oxides such as MnO, FeO, CoO and NiO are semiconductors and TiO and VO are metallic conductors. 3d-metal oxides - MnO, Fe1-xO, CoO and NiO have low conductivity that increase with temperature or have such large band gaps that become insulators. The electron-hole migration in these oxide ...
inorganic-chemistry-gp-i-alkali-metals
... Li here also shows an anomalous behaviour, when react with air it is the only metal to react with N2 present. Li + Air Li2O + Li3N the here also driving force is high lattice energy of product. Li3N + H2O LiOH + NH3 the production of ammonia makes this an important reaction. These are reaction ...
... Li here also shows an anomalous behaviour, when react with air it is the only metal to react with N2 present. Li + Air Li2O + Li3N the here also driving force is high lattice energy of product. Li3N + H2O LiOH + NH3 the production of ammonia makes this an important reaction. These are reaction ...
NOTES CHEMICAL REACTIONS:
... • Remember! In order for a reaction to take place you must produce a gas or a precipitate from 2 liquids. • Solubility rules tell us whether or not a ...
... • Remember! In order for a reaction to take place you must produce a gas or a precipitate from 2 liquids. • Solubility rules tell us whether or not a ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood
... 1. _______ matter is composed of particles called ___________ 2. Atoms form ______________ and come together in different ways to form _________________ and __________________ 3. ______ _____________ unit of an ___________ and maintain the ______________ of that element 4. ____________ _________ ...
... 1. _______ matter is composed of particles called ___________ 2. Atoms form ______________ and come together in different ways to form _________________ and __________________ 3. ______ _____________ unit of an ___________ and maintain the ______________ of that element 4. ____________ _________ ...
Metals & Metallurgy
... This model does not explain all properties of metals - for example, the highest melting points are found for group 6B. ...
... This model does not explain all properties of metals - for example, the highest melting points are found for group 6B. ...
Unit A Remediation Review
... 12. What are five clues that will allow you to conclude that a chemical change has occurred? 13. Describe what occurs in the following reaction types, the general equation and an example for each: a) Formation b) Decomposition c) Single Replacement d) Double Replacement e) Combustion 14. Write a bal ...
... 12. What are five clues that will allow you to conclude that a chemical change has occurred? 13. Describe what occurs in the following reaction types, the general equation and an example for each: a) Formation b) Decomposition c) Single Replacement d) Double Replacement e) Combustion 14. Write a bal ...
Synthesis/Decomposition Reactions
... How do you correctly identify, balance and predict the product(s) of synthesis reactions? How do you correctly identify, balance and predict the product(s) of decomposition reactions? ...
... How do you correctly identify, balance and predict the product(s) of synthesis reactions? How do you correctly identify, balance and predict the product(s) of decomposition reactions? ...
Liquid-feed flame spray pyrolysis

Liquid–feed flame spray pyrolysis (LF-FSP) is one of the most recent iterations in flame spray pyrolysis (FSP) powder production technology. FSP produces metal oxide powders from highly volatile gaseous metal chlorides that are decomposed/oxidized in hydrogen-oxygen flames to form nano-oxide powders. However, products made from FSP's vapor-phase process are limited to Al-, Ti-, Zr-, and Si-based oxides from their metal chlorides. Thus, interest in producing more complex materials required a new methodology, LF-FSP.LF-FSP, as invented at the University of Michigan, uses metalloorganic precursors such as metal carboxylates or alkoxides, not metal chlorides. Briefly, alcohol (typically ethanol) solutions containing 1–10 wt % loading of the target ceramic components as precursors are aerosolized with O2 into a quartz chamber and ignited with methane pilot torches. Initial combustion temperatures run 1500–2000 °C, depending on the processing conditions, generating nanopowder ""soot"". Temperatures drop to 300–500 °C over 1.5 m, equivalent to a 1000 °C quench in 100 ms leading to kinetic products and nanopowders that are unaggregated. Production rates can be 200 g/h when using wire-in-tube electrostatic precipitators operating at 10 kV. Typical powders have 15–100 nm average particle sizes (APS) with specific surface areas of 30–100 m2/g. LF-FSP technology can be used to produce mixed and single metal oxides easily from low cost starting materials in a single step without forming harmful byproducts like HCl, which forms when metal chlorides are used as precursors.