01 physical, technological and organizational bases of radiation
... living tissue contains radioactive substances. The radioactive characteristic is maintained throughout life. However, the normal radioactivity found in humans is nothing to worry about. Furthermore, nothing can be done to eliminate it. • The main radioactive materials in rocks are potassium-40, rubi ...
... living tissue contains radioactive substances. The radioactive characteristic is maintained throughout life. However, the normal radioactivity found in humans is nothing to worry about. Furthermore, nothing can be done to eliminate it. • The main radioactive materials in rocks are potassium-40, rubi ...
Introduction to Nuclear Radiation
... alpha particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interaction is with the electrons of the matter through which the radiation passes. Because of their much smaller mass, their velocity is much greater than alpha particles and since their ...
... alpha particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interaction is with the electrons of the matter through which the radiation passes. Because of their much smaller mass, their velocity is much greater than alpha particles and since their ...
Chapter 9 Nuclear Radiation
... The total mass of the products in this reaction is slightly less than the mass of the starting materials. The missing mass has been converted into energy, consistent with the famous equation derived by Albert Einstein, E = mc2. E is the energy released, m is the mass lost, and c is the speed of ligh ...
... The total mass of the products in this reaction is slightly less than the mass of the starting materials. The missing mass has been converted into energy, consistent with the famous equation derived by Albert Einstein, E = mc2. E is the energy released, m is the mass lost, and c is the speed of ligh ...
Radiation Safety - 7
... inert gas inside the tube. The radiation enters the tube ionizing the gas thus creating a current flow. The amount of radiation is proportional to the current flow. ...
... inert gas inside the tube. The radiation enters the tube ionizing the gas thus creating a current flow. The amount of radiation is proportional to the current flow. ...
Radioactivity Revision Questions Decay – Nucleus
... Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting particles or waves from the nucl ...
... Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting particles or waves from the nucl ...
nuclear radiation, continued
... radioactive decay? 〉It is impossible to predict the moment when any particular nucleus will decay, but it is possible to predict the time required for half of the nuclei in a given radioactive sample to decay. half-life: the time required for half of a sample of a radioactive isotope to break down b ...
... radioactive decay? 〉It is impossible to predict the moment when any particular nucleus will decay, but it is possible to predict the time required for half of the nuclei in a given radioactive sample to decay. half-life: the time required for half of a sample of a radioactive isotope to break down b ...
do physics online from quanta to quarks radioactivity
... The need to account for the energy distribution of electrons emitted in beta decay (figure 1) and to satisfy the laws of conservation of energy, linear and angular momentum, Austrian physicist Wolfgang Pauli in 1930 proposed that a neutral particle was emitted along with the particle. This partic ...
... The need to account for the energy distribution of electrons emitted in beta decay (figure 1) and to satisfy the laws of conservation of energy, linear and angular momentum, Austrian physicist Wolfgang Pauli in 1930 proposed that a neutral particle was emitted along with the particle. This partic ...
catch some rays: alpha, beta, gamma (modified for adeed)
... Beta particle decay emits either an electron or a positron. When an electron is emitted, the number of neutrons is decreased by one and the number of protons increased by one. When a positron is emitted, the number of protons is decreased by one and the number of neutrons increased by one. Beta part ...
... Beta particle decay emits either an electron or a positron. When an electron is emitted, the number of neutrons is decreased by one and the number of protons increased by one. When a positron is emitted, the number of protons is decreased by one and the number of neutrons increased by one. Beta part ...
Nuclear Notation
... • First to discover that Uranium is radioactive – Left Uranium Ore in his desk next to photographic paper. – When developed the film had an image on it from the Uranium decaying ...
... • First to discover that Uranium is radioactive – Left Uranium Ore in his desk next to photographic paper. – When developed the film had an image on it from the Uranium decaying ...
Introduction to Nuclear Radiation
... particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interaction is with the electrons of the matter through which the radiation passes. Because of their much smaller mass, their velocity is much greater than alpha particles and since their charg ...
... particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interaction is with the electrons of the matter through which the radiation passes. Because of their much smaller mass, their velocity is much greater than alpha particles and since their charg ...
Nuclear Chemistry - Duplin County Schools
... Nuclear Decays and Reactions • When an unstable nucleus decays, particles and energy called nuclear radiation are emitted from it. • The three types of nuclear radiation are alpha, beta, and gamma radiation. • Alpha and beta radiation are particles. Gamma radiation is an electromagnetic wave. ...
... Nuclear Decays and Reactions • When an unstable nucleus decays, particles and energy called nuclear radiation are emitted from it. • The three types of nuclear radiation are alpha, beta, and gamma radiation. • Alpha and beta radiation are particles. Gamma radiation is an electromagnetic wave. ...
chapter 5 Radioactivity
... Atoms are electrically neutral, containing the same number of protons and electrons. If an atom gains or loses electrons, and thus becomes negatively or positively charged, it is no longer an atom but an ion. Atoms of the same element have the same number of protons and electrons, but can have diffe ...
... Atoms are electrically neutral, containing the same number of protons and electrons. If an atom gains or loses electrons, and thus becomes negatively or positively charged, it is no longer an atom but an ion. Atoms of the same element have the same number of protons and electrons, but can have diffe ...
7.2 - Moodle
... • α-particle is the least penetrating, it is stopped by a thick sheet of paper, or by the skin, or after traveling few cm in air. • β particles are penetrating, but they are stopped by few millimeters in aluminum or other metal • γ particles are very penetrating. They are never completely stopped, b ...
... • α-particle is the least penetrating, it is stopped by a thick sheet of paper, or by the skin, or after traveling few cm in air. • β particles are penetrating, but they are stopped by few millimeters in aluminum or other metal • γ particles are very penetrating. They are never completely stopped, b ...
Absorption and Biological Effects of Ionising Radiation
... uranium. The process of alpha decay transforms one element into another, decreasing the atomic mass (or nuclear number) by four. Because of their large mass alpha particles have short range of only a few centimetres in air. Alpha radiations may only penetrate as deep as the surface of the skin. Howe ...
... uranium. The process of alpha decay transforms one element into another, decreasing the atomic mass (or nuclear number) by four. Because of their large mass alpha particles have short range of only a few centimetres in air. Alpha radiations may only penetrate as deep as the surface of the skin. Howe ...
Study of Neutron and Gamma Radiation Protective
... a variety of materials such as lead, iron, graphite, water, polyethylene or concrete are used. Among these materials, Concrete is one of the best and most widely used materials for manufacture of Gamma and neutron radiation shield, because in addition to having the proper Structural properties of th ...
... a variety of materials such as lead, iron, graphite, water, polyethylene or concrete are used. Among these materials, Concrete is one of the best and most widely used materials for manufacture of Gamma and neutron radiation shield, because in addition to having the proper Structural properties of th ...
Chapter 29
... • Except for light nuclei, the binding energy is about 8 MeV per nucleon • The curve peaks in the vicinity of A = 60 • Nuclei with mass numbers greater than or less than 60 are not as strongly bound as those near the middle of the periodic table • The curve is slowly varying at A > 40, suggesting th ...
... • Except for light nuclei, the binding energy is about 8 MeV per nucleon • The curve peaks in the vicinity of A = 60 • Nuclei with mass numbers greater than or less than 60 are not as strongly bound as those near the middle of the periodic table • The curve is slowly varying at A > 40, suggesting th ...
Basics of nuclear physics
... in opposite direction Only for photons with high energies interacting with high-atomic-number elements ...
... in opposite direction Only for photons with high energies interacting with high-atomic-number elements ...
Chapter 30: Nuclear Physics What will we learn in this chapter?
... Can be shielded with thick clothing, used in medical applications. ...
... Can be shielded with thick clothing, used in medical applications. ...
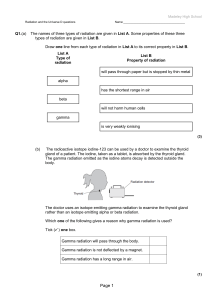
Page 1 - Madeley High School
... Which one of these three stars has the lifecycle shown in part (a)(i)?............................................ Give a reason for your answer................................................................................................ ...
... Which one of these three stars has the lifecycle shown in part (a)(i)?............................................ Give a reason for your answer................................................................................................ ...
Learning Check Key - Mayfield City Schools
... Radiation Protection Radiation protection requires • paper and clothing for alpha particles • a lab coat or gloves for beta particles • a lead shield or a thick concrete wall for gamma rays • limiting the amount of time spent near a radioactive source • increasing the distance from the source ...
... Radiation Protection Radiation protection requires • paper and clothing for alpha particles • a lab coat or gloves for beta particles • a lead shield or a thick concrete wall for gamma rays • limiting the amount of time spent near a radioactive source • increasing the distance from the source ...
Week 13 Chemistry
... Energy Nuclear Energy Life The Sun is an example of nuclear chemistry at work Medicine X-rays, MRI ...
... Energy Nuclear Energy Life The Sun is an example of nuclear chemistry at work Medicine X-rays, MRI ...
Chapter 2, section 4 Formation of Elements
... Mass # = number of protons + number of neutrons Atomic # = number of protons ...
... Mass # = number of protons + number of neutrons Atomic # = number of protons ...
Ch.7 Summary Notes
... Nuclear fusion is a nuclear reaction in which small nuclei combine to produce a larger nucleus. Other subatomic particles as well as energy are released in this process. Fusion occurs at the core of the Sun and other stars where sufficient pressure and high temperatures cause isotopes of hydrogen to ...
... Nuclear fusion is a nuclear reaction in which small nuclei combine to produce a larger nucleus. Other subatomic particles as well as energy are released in this process. Fusion occurs at the core of the Sun and other stars where sufficient pressure and high temperatures cause isotopes of hydrogen to ...
Introduction to Radiation Physics, Quantities and Units
... nanometers can remove electrons from the outer atomic shells. • This process produces ions. • Ions can interact with living tissue to produce biological damage. • A major source of ionizing radiation is nuclear transformation. ...
... nanometers can remove electrons from the outer atomic shells. • This process produces ions. • Ions can interact with living tissue to produce biological damage. • A major source of ionizing radiation is nuclear transformation. ...
Fallout shelter

A fallout shelter is an enclosed space specially designed to protect occupants from radioactive debris or fallout resulting from a nuclear explosion. Many such shelters were constructed as civil defense measures during the Cold War.During a nuclear explosion, matter vaporized in the resulting fireball is exposed to neutrons from the explosion, absorbs them, and becomes radioactive. When this material condenses in the rain, it forms dust and light sandy materials that resembles ground pumice. The fallout emits alpha and beta particles, as well as gamma rays.Much of this highly radioactive material falls to earth, subjecting anything within the line of sight to radiation, becoming a significant hazard. A fallout shelter is designed to allow its occupants to minimize exposure to harmful fallout until radioactivity has decayed to a safer level.