2nd Semester Exam Review
... • Number of particles decreases in a reaction • Dissolving of gas in a solvent • Decrease in temperature ...
... • Number of particles decreases in a reaction • Dissolving of gas in a solvent • Decrease in temperature ...
AP Chem Stoichiometry Topic#4 Questions WS Name: Date: Per
... (4) Based on the structural formula, calculate the percentage of carbon by mass present in the compound. (5) The diagram represents the collection of elements formed by a decomposition reaction. (a) If the blue spheres represent N atoms and the red ones represent O atoms, what was the empirical form ...
... (4) Based on the structural formula, calculate the percentage of carbon by mass present in the compound. (5) The diagram represents the collection of elements formed by a decomposition reaction. (a) If the blue spheres represent N atoms and the red ones represent O atoms, what was the empirical form ...
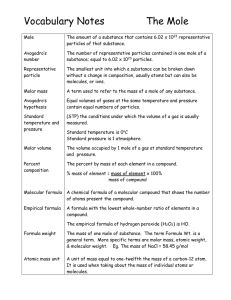
Vocabulary Notes
... The mass of one mole of substance. The term Formula Wt. is a general term. More specific terms are molar mass, atomic weight, & molecular weight. Eg. The mass of NaCl = 58.45 g/mol ...
... The mass of one mole of substance. The term Formula Wt. is a general term. More specific terms are molar mass, atomic weight, & molecular weight. Eg. The mass of NaCl = 58.45 g/mol ...
HonorsChem.final.rev.probs
... 33. A sample of SO2 has a pressure of 950 mm Hg in a volume of 460 mL. The sample is moved to a new flask in which the pressure of the gas is now 400 mm Hg. What is the volume of the new flask? ...
... 33. A sample of SO2 has a pressure of 950 mm Hg in a volume of 460 mL. The sample is moved to a new flask in which the pressure of the gas is now 400 mm Hg. What is the volume of the new flask? ...
Electrostatics Review
... Two positively charged masses are separated by a distance, r. Which statement best describes the gravitational and electrostatic forces between the two masses? (A) Both forces are attractive. (B) Both forces are repulsive. (C) The gravitational force is repulsive and the electrostatic force is attr ...
... Two positively charged masses are separated by a distance, r. Which statement best describes the gravitational and electrostatic forces between the two masses? (A) Both forces are attractive. (B) Both forces are repulsive. (C) The gravitational force is repulsive and the electrostatic force is attr ...
chemI.final.rev.probs
... a) Determine the number of grams of KOH that will be produced when 97 g of potassium are used. b) Determine the number of liters of H2 gas that will be produced when 6.5 X 1024 molecules of water ...
... a) Determine the number of grams of KOH that will be produced when 97 g of potassium are used. b) Determine the number of liters of H2 gas that will be produced when 6.5 X 1024 molecules of water ...
March: I`ve got two worlds on a string
... +q and the smaller mass has charge –q. Making the assumption that the masses of the spheres are orders of magnitude greater than the mobile electrons, we can say that the charges are at all times in equilibrium. When the spheres are a distance s apart, the potentials due to the separation of the cha ...
... +q and the smaller mass has charge –q. Making the assumption that the masses of the spheres are orders of magnitude greater than the mobile electrons, we can say that the charges are at all times in equilibrium. When the spheres are a distance s apart, the potentials due to the separation of the cha ...
Test 1 - Al Akhawayn University
... (a) What beat frequency does he observe between the tuning fork and its echo? (b) How fast must he walk away from the wall to observe a beat frequency of 5Hz? 2) Two small spheres each of mass m = 2.00 g are suspended by light strings 10.0 cm in length (Figure 1). A uniform electric field is applied ...
... (a) What beat frequency does he observe between the tuning fork and its echo? (b) How fast must he walk away from the wall to observe a beat frequency of 5Hz? 2) Two small spheres each of mass m = 2.00 g are suspended by light strings 10.0 cm in length (Figure 1). A uniform electric field is applied ...
Document
... Two positively charged masses are separated by a distance, r. Which statement best describes the gravitational and electrostatic forces between the two masses? (A) Both forces are attractive. (B) Both forces are repulsive. (C) The gravitational force is repulsive and the electrostatic force is attr ...
... Two positively charged masses are separated by a distance, r. Which statement best describes the gravitational and electrostatic forces between the two masses? (A) Both forces are attractive. (B) Both forces are repulsive. (C) The gravitational force is repulsive and the electrostatic force is attr ...