1 Quantitative chemistry - Pearson Schools and FE Colleges
... 1.2.5 Determine the empirical formula from the percentage composition or from other experimental data. 1.2.6 Determine the molecular formula when given both the empirical formula and experimental data. ...
... 1.2.5 Determine the empirical formula from the percentage composition or from other experimental data. 1.2.6 Determine the molecular formula when given both the empirical formula and experimental data. ...
Unit- 5.pmd
... adsorption. During adsorption, there is always a decrease in residual forces of the surface, i.e., there is decrease in surface energy which appears as heat. Adsorption, therefore, is invariably an exothermic process. In other words, ∆H of adsorption is always negative. When a gas is adsorbed, the f ...
... adsorption. During adsorption, there is always a decrease in residual forces of the surface, i.e., there is decrease in surface energy which appears as heat. Adsorption, therefore, is invariably an exothermic process. In other words, ∆H of adsorption is always negative. When a gas is adsorbed, the f ...
Unit 13 Chap. 14 Solution Chemistry Lecture Notes
... SUBSTANCES THAT ARE NOT SOLUBLE IN EACH OTHER ARE IMMISCIBLE. (OIL AND WATER) TWO SUBSTANCES THAT ARE MUTUALLY SOLUBLE IN ALL PROPORTIONS ARE SAID TO BE MISCIBLE. HENRY’S LAW - THE SOLUBILITY OF A GAS IN A LIQUID IS DIRECTLY PROPORTIONAL TO THE PARTIAL PRESSURE OF THAT GAS ON THE SURFACE OF THE LIQU ...
... SUBSTANCES THAT ARE NOT SOLUBLE IN EACH OTHER ARE IMMISCIBLE. (OIL AND WATER) TWO SUBSTANCES THAT ARE MUTUALLY SOLUBLE IN ALL PROPORTIONS ARE SAID TO BE MISCIBLE. HENRY’S LAW - THE SOLUBILITY OF A GAS IN A LIQUID IS DIRECTLY PROPORTIONAL TO THE PARTIAL PRESSURE OF THAT GAS ON THE SURFACE OF THE LIQU ...
الشريحة 1
... themselves on the surface of the NaCl crystals. The +ve end of H2O dipole is oriented toward the Clions, and the –ve end of the H2O dipole is oriented toward the Na+ ions. The ion-dipole attractions between the ions and H2O molecules are strong enough to pull the ions from their positions in the cry ...
... themselves on the surface of the NaCl crystals. The +ve end of H2O dipole is oriented toward the Clions, and the –ve end of the H2O dipole is oriented toward the Na+ ions. The ion-dipole attractions between the ions and H2O molecules are strong enough to pull the ions from their positions in the cry ...
Moles 2016
... It started with the atomic masses for the elements. For example, carbon has an average atomic mass of 12.01 amu, meaning that one atom of carbon has a mass of 12.01 amu. The scientists wanted to be able to use the convenient atomic masses for the elements, but on a larger scale. So they decide ...
... It started with the atomic masses for the elements. For example, carbon has an average atomic mass of 12.01 amu, meaning that one atom of carbon has a mass of 12.01 amu. The scientists wanted to be able to use the convenient atomic masses for the elements, but on a larger scale. So they decide ...
CHEMISTRY - careerpoint.ac.in
... “This law states that, a given compound, wherever obtained and however prepared contain its component elements in a fixed ratio by weight.” As for example, water obtained from any source contains hydrogen and oxygen combined in a ratio of 2 : 16 = 1 : 8 by weight. (c) The law of multiple proportion ...
... “This law states that, a given compound, wherever obtained and however prepared contain its component elements in a fixed ratio by weight.” As for example, water obtained from any source contains hydrogen and oxygen combined in a ratio of 2 : 16 = 1 : 8 by weight. (c) The law of multiple proportion ...
Unit3_Stoichiometry_vs2
... so the temperature increases. • b–c This is the melting point. The vibrations are sufficiently energetic for the molecules to move away from their fixed positions and form liquid. Energy added during this stage is used to break the inter-particle forces, not to raise the kinetic energy, so the tempe ...
... so the temperature increases. • b–c This is the melting point. The vibrations are sufficiently energetic for the molecules to move away from their fixed positions and form liquid. Energy added during this stage is used to break the inter-particle forces, not to raise the kinetic energy, so the tempe ...
Can atoms be counted or measured
... Save this for another day Objective 21 – Determine the empirical formula of a compound when given the percent composition. A. There are 4 steps for determining the empirical formula when given the percent composition. 1. Convert the percent composition of each substance to grams. 2. Convert the gra ...
... Save this for another day Objective 21 – Determine the empirical formula of a compound when given the percent composition. A. There are 4 steps for determining the empirical formula when given the percent composition. 1. Convert the percent composition of each substance to grams. 2. Convert the gra ...
Physical Chemistry II
... conditions of temperature and pressure. In electrochemistry, we focus on chemical systems that involve the transfer of electrons or charged species i.e. oxidation – reduction reaction s.. Nuclear chemistry looks at processes that are associated with the nucleus. Nuclei of certain isotopes are unstab ...
... conditions of temperature and pressure. In electrochemistry, we focus on chemical systems that involve the transfer of electrons or charged species i.e. oxidation – reduction reaction s.. Nuclear chemistry looks at processes that are associated with the nucleus. Nuclei of certain isotopes are unstab ...
Chapter 7: Solutions
... or dissolved NaCl, requires that solute particles be able to interact with the solvent molecules through noncovalent interactions. ...
... or dissolved NaCl, requires that solute particles be able to interact with the solvent molecules through noncovalent interactions. ...
Physical Chemistry 2.pdf
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
Gas Laws
... What is a solid – solid solution of two or more metals called? alloy A mixture in which the particles are so small that they will not reflect the “light” from a laser are called solution. A solution that contains a large amount of solute per amount of solvent is called a concentrated solution. What ...
... What is a solid – solid solution of two or more metals called? alloy A mixture in which the particles are so small that they will not reflect the “light” from a laser are called solution. A solution that contains a large amount of solute per amount of solvent is called a concentrated solution. What ...
Gas Laws
... What is a solid – solid solution of two or more metals called? alloy A mixture in which the particles are so small that they will not reflect the “light” from a laser are called solution. A solution that contains a large amount of solute per amount of solvent is called a concentrated solution. What ...
... What is a solid – solid solution of two or more metals called? alloy A mixture in which the particles are so small that they will not reflect the “light” from a laser are called solution. A solution that contains a large amount of solute per amount of solvent is called a concentrated solution. What ...
C - Thierry Karsenti
... contact of molecules of different types thereby facilitating chemical reactions. The study of solutions is important as most reaction, be it in the laboratory, industry or life systems take place in solution. They also provide a convenient and accurate means by which we can introduce small quantitie ...
... contact of molecules of different types thereby facilitating chemical reactions. The study of solutions is important as most reaction, be it in the laboratory, industry or life systems take place in solution. They also provide a convenient and accurate means by which we can introduce small quantitie ...
Porous silicon-based nanostructured microparticles as degradable
... 1(3)): A solution of 11-undecenylamine (0.677 g, 4 mmol) in 30 mL of anhydrous CH2Cl2 was prepared and cooled in an ice bath. In a separate flask, 9-fluorenylmethoxycarbonyl chloride (1.04 g, 4 mmol) was dissolved in a small amount of anhydrous CH2Cl2 and was slowly added to the solution of 11undece ...
... 1(3)): A solution of 11-undecenylamine (0.677 g, 4 mmol) in 30 mL of anhydrous CH2Cl2 was prepared and cooled in an ice bath. In a separate flask, 9-fluorenylmethoxycarbonyl chloride (1.04 g, 4 mmol) was dissolved in a small amount of anhydrous CH2Cl2 and was slowly added to the solution of 11undece ...
1 The Mole 6.02 X 10 23
... AND 1 mole = molar mass (grams) • You can convert atoms/molecules to moles and then moles to grams! (Two step ...
... AND 1 mole = molar mass (grams) • You can convert atoms/molecules to moles and then moles to grams! (Two step ...
Stoichiometry, % Comp, Empirical & Molecular Formula
... So % abundance of N-14 = 99.668907% % abundance of N-15 = 0.3310926% ...
... So % abundance of N-14 = 99.668907% % abundance of N-15 = 0.3310926% ...
Jon Abbatt - Earth, Atmospheric, and Planetary Physics
... To some degree, this depends on the setting. For example, in a large dust storm coming off the Gobi desert, the total particle surface area and mass will be overwhelmingly dominated by mineral dust. But, as this mineral dust “ages”, it will pick up a thin mixed sulfate/organic coating in addition to ...
... To some degree, this depends on the setting. For example, in a large dust storm coming off the Gobi desert, the total particle surface area and mass will be overwhelmingly dominated by mineral dust. But, as this mineral dust “ages”, it will pick up a thin mixed sulfate/organic coating in addition to ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... 19. The molarity of a solution prepared by diluting 43.72 mL of 5.005 M aqueous K2Cr2O7 to 500 mL is __________. (a). 57.2 (b). 0.0044 (c). 0.438 (d). 0.0879 Explanation: You should use the M1V1 = M2V2 formula here. It is the only formula that you should use here since this is just a dilution of the ...
... 19. The molarity of a solution prepared by diluting 43.72 mL of 5.005 M aqueous K2Cr2O7 to 500 mL is __________. (a). 57.2 (b). 0.0044 (c). 0.438 (d). 0.0879 Explanation: You should use the M1V1 = M2V2 formula here. It is the only formula that you should use here since this is just a dilution of the ...
File
... continues. These 2 processes continue at the same rate (speed), therefore no net change in concentration occurs and the solution is in “dynamic equilibrium”. The rate of dissolving = the rate of recrystallization ...
... continues. These 2 processes continue at the same rate (speed), therefore no net change in concentration occurs and the solution is in “dynamic equilibrium”. The rate of dissolving = the rate of recrystallization ...
Dissociation
... — Learn to use your reference tables — it’s fun and if you take advantage of this special limited time offer, it’s absolutely free — The guidelines are useful in helping to predict what will happen if the solutions of two different soluble compounds are mixed — If the mixing results in a combination ...
... — Learn to use your reference tables — it’s fun and if you take advantage of this special limited time offer, it’s absolutely free — The guidelines are useful in helping to predict what will happen if the solutions of two different soluble compounds are mixed — If the mixing results in a combination ...
Full text in PDF form
... Here we have identi ed the general uid quantities of the previous section with those following from the dynamics of a Maxwell-Boltzmann gas. Note that the energy-momentum tensor T ik in Eqs. (30) and (41) does not coincide with the tensor T(ike ) introduced in Eq. (2). The exact integral expressio ...
... Here we have identi ed the general uid quantities of the previous section with those following from the dynamics of a Maxwell-Boltzmann gas. Note that the energy-momentum tensor T ik in Eqs. (30) and (41) does not coincide with the tensor T(ike ) introduced in Eq. (2). The exact integral expressio ...
The Mole - cloudfront.net
... 2. Calculate the molar mass of this formula. 3. Obtain the molar mass of the compound in question. 4. Divide this molar mass by the molar mass of the empirical formula. 5. This is the multiplier used to obtain the molecular formula from the empirical ...
... 2. Calculate the molar mass of this formula. 3. Obtain the molar mass of the compound in question. 4. Divide this molar mass by the molar mass of the empirical formula. 5. This is the multiplier used to obtain the molecular formula from the empirical ...
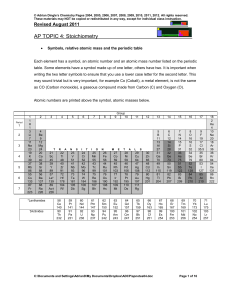
Mole Concept and Stoichiometry
... One interpretation : A specific number of particles When a quantity of particles is to be described, mole is a grouping unit analogous to groupings such as pair, dozen or gross, in that all of these words represent specific numbers of objects. The main difference between the mole and the other group ...
... One interpretation : A specific number of particles When a quantity of particles is to be described, mole is a grouping unit analogous to groupings such as pair, dozen or gross, in that all of these words represent specific numbers of objects. The main difference between the mole and the other group ...