Exam1_actual
... 8. (6 points) Draw Lineweaver-Burk plots for each of the three types of non-covalent inhibition at 2 concentrations of inhibitor. You must draw the chemical reaction for each type of inhibition (E + I or E + S, etc.) and give the name of the type of inhibition under each of the plots. ...
... 8. (6 points) Draw Lineweaver-Burk plots for each of the three types of non-covalent inhibition at 2 concentrations of inhibitor. You must draw the chemical reaction for each type of inhibition (E + I or E + S, etc.) and give the name of the type of inhibition under each of the plots. ...
Biosynthesis of non-amino acids from amino acid precursors
... Major problem is secondary skin infections by destruction. Scarring and loss of extremeties. Sometimes have craving for blood (?). Old cells cleared by spleen and degraded Heme degraded to bilirubin, has yellow color Bilirubin deleiverd to liver bound to serum albumin, and conjugated to two ...
... Major problem is secondary skin infections by destruction. Scarring and loss of extremeties. Sometimes have craving for blood (?). Old cells cleared by spleen and degraded Heme degraded to bilirubin, has yellow color Bilirubin deleiverd to liver bound to serum albumin, and conjugated to two ...
Biocatalysis - School of Chemical Sciences
... like high catalytic efficiency and mild operational conditions, are also very attractive in commercial applications. The characteristics of limited operating regions, substrate or product inhibition, and reactions in aqueous solutions have often been considered as the most serious drawbacks of biocat ...
... like high catalytic efficiency and mild operational conditions, are also very attractive in commercial applications. The characteristics of limited operating regions, substrate or product inhibition, and reactions in aqueous solutions have often been considered as the most serious drawbacks of biocat ...
Enzymes - Catawba County Schools
... Catalyst- substance that speeds up the rate of a chemical reaction. They lower the reactions activation energy. Enzymes-proteins that act as biological catalysts. Enzymes are not used up during the reaction and can be used over and over again. Enzymes can usually be identified by the "ase" ending in ...
... Catalyst- substance that speeds up the rate of a chemical reaction. They lower the reactions activation energy. Enzymes-proteins that act as biological catalysts. Enzymes are not used up during the reaction and can be used over and over again. Enzymes can usually be identified by the "ase" ending in ...
Exam 4, 2015 - Biochemistry at CSU, Stanislaus
... 8. Anaplerotic reactions, such as the conversion of pyruvate to oxaloacetate, are useful to the citric acid cycle because they A. generate a steady supply of intermediates for the citric acid cycle. B. produce molecules needed to regulate the citric acid cycle. C. link the citric acid cycle to the g ...
... 8. Anaplerotic reactions, such as the conversion of pyruvate to oxaloacetate, are useful to the citric acid cycle because they A. generate a steady supply of intermediates for the citric acid cycle. B. produce molecules needed to regulate the citric acid cycle. C. link the citric acid cycle to the g ...
Anatomy and Physiology Chapter #4
... Enzymes control the rates of all the metabolic reactions of the cell. Enzymes are complex molecule (PROTEIN) that function to lower the activation energy of a reaction so it may begin and proceed more quickly. Because they do this, enzymes are called catalysis. The substances the enzymes act on are ...
... Enzymes control the rates of all the metabolic reactions of the cell. Enzymes are complex molecule (PROTEIN) that function to lower the activation energy of a reaction so it may begin and proceed more quickly. Because they do this, enzymes are called catalysis. The substances the enzymes act on are ...
Group 6 - Purdue Genomics Wiki
... Matched orthologs in 5 other plant genomes. Image from: http://pdb.rcsb.org ...
... Matched orthologs in 5 other plant genomes. Image from: http://pdb.rcsb.org ...
Answers - Shelton State
... carries oxygen. 10. Which of the following are macromolecules? proteins and carbohydrates but not lipids 11. What is the net charge on cysteine, pI=5.1, when the pH=6.3? negative Which way will it move during electrophoresis? Toward the positive electrode. 12. The names of enzymes often identify the ...
... carries oxygen. 10. Which of the following are macromolecules? proteins and carbohydrates but not lipids 11. What is the net charge on cysteine, pI=5.1, when the pH=6.3? negative Which way will it move during electrophoresis? Toward the positive electrode. 12. The names of enzymes often identify the ...
Metabolism & Enzymes
... a lot of stored energy in each bond most energy stored in 3rd Pi 3rd Pi is hardest group to keep bonded to molecule ...
... a lot of stored energy in each bond most energy stored in 3rd Pi 3rd Pi is hardest group to keep bonded to molecule ...
3.2 Carbohydrates, Lipids, and Proteins
... – Ex: glycerol + 3 fatty acids triglyceride + 3 water – Ex: amino acids (many) protein + water (many) ...
... – Ex: glycerol + 3 fatty acids triglyceride + 3 water – Ex: amino acids (many) protein + water (many) ...
Non-Selective Inhibition of Trypanosoma cruzi GAPDH and rabbit
... employed in kinetics parameters determination of enzymes belonging to trypanosomatids. In this work, we report a calorimetric assay for glycosomal glyceraldehyde-3-phophate dehydrogenase enzyme (EC 1.2.1.12 - GAPDH) an important enzyme in the life cycle of the protozoan parasite Trypanosoma cruzi, t ...
... employed in kinetics parameters determination of enzymes belonging to trypanosomatids. In this work, we report a calorimetric assay for glycosomal glyceraldehyde-3-phophate dehydrogenase enzyme (EC 1.2.1.12 - GAPDH) an important enzyme in the life cycle of the protozoan parasite Trypanosoma cruzi, t ...
Industrial Biotechnology
... • The final product of metabolic pathway inhibits the action of earlier enzymes (usually the first) of that sequence. • The inhibitor and the substrate need not resemble each other, hence the inhibition is often called allosteric. • In case of isosteic inhibition the inhibitor and substrate have the ...
... • The final product of metabolic pathway inhibits the action of earlier enzymes (usually the first) of that sequence. • The inhibitor and the substrate need not resemble each other, hence the inhibition is often called allosteric. • In case of isosteic inhibition the inhibitor and substrate have the ...
Ch. 2 The Chemistry of Life
... Carbohydrates - compounds made up of carbon, hydrogen, & oxygen atoms Living things use carbohydrates as their main source of energy ...
... Carbohydrates - compounds made up of carbon, hydrogen, & oxygen atoms Living things use carbohydrates as their main source of energy ...
Discussion prompts
... 1. What is an enzyme? What kind of molecule is an enzyme? Give examples of biological processes that use enzymes. [An enzyme is a biological molecule that serves as a catalyst to help biochemical reactions. The vast majority of enzymes are proteins, although other types of molecules such as RNA can ...
... 1. What is an enzyme? What kind of molecule is an enzyme? Give examples of biological processes that use enzymes. [An enzyme is a biological molecule that serves as a catalyst to help biochemical reactions. The vast majority of enzymes are proteins, although other types of molecules such as RNA can ...
No Slide Title
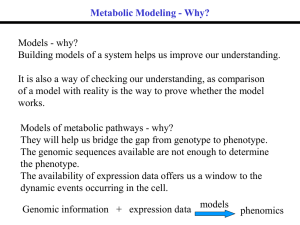
... What was really meant was that the rate of a pathway could be altered only by changing the activity of one particular enzyme. Despite the fact that experimental data has often contradicted this concept, much of the work on the control of metabolism has been centred around the search for potential re ...
... What was really meant was that the rate of a pathway could be altered only by changing the activity of one particular enzyme. Despite the fact that experimental data has often contradicted this concept, much of the work on the control of metabolism has been centred around the search for potential re ...
Proteolytic Enzymes from Extremely Halophilic Bacteria
... Proteolysis was also demonstrated by growing the bacteria on the same medium solidified with agar. The agar plates were inoculated in the middle with a loop so that one colony was obtained. The enzyme activity was then determined by measuring the clear zones around the colonies. Marked differences i ...
... Proteolysis was also demonstrated by growing the bacteria on the same medium solidified with agar. The agar plates were inoculated in the middle with a loop so that one colony was obtained. The enzyme activity was then determined by measuring the clear zones around the colonies. Marked differences i ...
Final Report
... of Noxo1 is that they have identical sequences with the exception of the gamma form having a five amino acid insert in the lipid-binding domain. Even though they are sequentially very similar, the two forms have differences in how active the enzyme complex is, where the proteins are located in a cel ...
... of Noxo1 is that they have identical sequences with the exception of the gamma form having a five amino acid insert in the lipid-binding domain. Even though they are sequentially very similar, the two forms have differences in how active the enzyme complex is, where the proteins are located in a cel ...
Document
... 2. It is often convenient to assay an enzyme reaction by coupling it with another enzyme to form a product that is easily quantified. An example is the assay of pyruvate kinase by coupling it with lactate dehydrogenase. Phosphoenolpyruvate + ADP + H+ → pyruvate + ATP Pyruvate + NADH + H+ → lactate + ...
... 2. It is often convenient to assay an enzyme reaction by coupling it with another enzyme to form a product that is easily quantified. An example is the assay of pyruvate kinase by coupling it with lactate dehydrogenase. Phosphoenolpyruvate + ADP + H+ → pyruvate + ATP Pyruvate + NADH + H+ → lactate + ...
doc NUR1 200 Midterm 2006
... 38. Which of the following statements about allosteric control of enzymatic activity is false? A) Allosteric effectors give rise to sigmoidal V0 vs. [S] kinetic plots. B) Allosteric proteins are generally composed of several subunits. C) An effector may either inhibit or activate an enzyme. D) Bindi ...
... 38. Which of the following statements about allosteric control of enzymatic activity is false? A) Allosteric effectors give rise to sigmoidal V0 vs. [S] kinetic plots. B) Allosteric proteins are generally composed of several subunits. C) An effector may either inhibit or activate an enzyme. D) Bindi ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.