Biomolecules Review
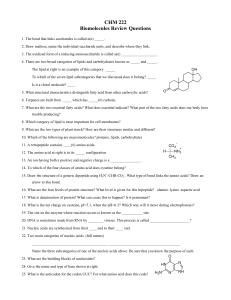
... 14. To which of the four classes of amino acid does cysteine belong? 15. Draw the structure of a generic dipeptide using H3N+-CHR-CO2-. What type of bond links the amino acids? Draw an arrow to this bond. 16. What are the four levels of protein structure? What level is given for this tripeptide? ala ...
... 14. To which of the four classes of amino acid does cysteine belong? 15. Draw the structure of a generic dipeptide using H3N+-CHR-CO2-. What type of bond links the amino acids? Draw an arrow to this bond. 16. What are the four levels of protein structure? What level is given for this tripeptide? ala ...
serine proteases
... 1. Serine proteases are members of a large group of proteolytic enzymes, all of which have at their active site a serine residue which plays a crucial part in the enzymic activity. All cleave peptide bonds, by a similar mechanism of action. They differ in their specificity and regulation. 2. Serine ...
... 1. Serine proteases are members of a large group of proteolytic enzymes, all of which have at their active site a serine residue which plays a crucial part in the enzymic activity. All cleave peptide bonds, by a similar mechanism of action. They differ in their specificity and regulation. 2. Serine ...
Exam 2 Review Sheet - Iowa State University
... 1.) Can you apply the concepts of chemical energy and energy states in explaining the functions of ATP and of enzymes in cellular chemistry? 2.) Can you describe the role of enzymes in chemical reactions? 3.) Can you determine whether a reaction is spontaneous or not based on free energy values? 4.) ...
... 1.) Can you apply the concepts of chemical energy and energy states in explaining the functions of ATP and of enzymes in cellular chemistry? 2.) Can you describe the role of enzymes in chemical reactions? 3.) Can you determine whether a reaction is spontaneous or not based on free energy values? 4.) ...
Molecules of Life
... Polypeptide: Many amino acids Tripeptide: Three amino acids Dipeptide: Two amino acids ...
... Polypeptide: Many amino acids Tripeptide: Three amino acids Dipeptide: Two amino acids ...
Document
... If bB is very large, even at low pB, A will be very small. The reaction of A will be greatly retarded. The impurities with high b is catalyst poison. ...
... If bB is very large, even at low pB, A will be very small. The reaction of A will be greatly retarded. The impurities with high b is catalyst poison. ...
Enzymes in the Diagnosis of Pathology
... Ceruoplasmin is a plasma protein with ferroxidase activity . Normal values are 280-570 units . This enzyme is used to establish the presence of Wilson’s disease ( cirrhotic liver ) in which the levels are decreased from normal . Cholinesterase found in blood serum is usually called type ll cholinest ...
... Ceruoplasmin is a plasma protein with ferroxidase activity . Normal values are 280-570 units . This enzyme is used to establish the presence of Wilson’s disease ( cirrhotic liver ) in which the levels are decreased from normal . Cholinesterase found in blood serum is usually called type ll cholinest ...
tb_ch21
... 21.36 d - FFF Statements: (1) The active site of an enzyme always contains one or more metal atoms. (2) The water-soluble vitamins are the B vitamins and vitamins A and C. (3) An enzyme’s turnover number is the rate at which it is degraded and resynthesized within the human body. a) All three statem ...
... 21.36 d - FFF Statements: (1) The active site of an enzyme always contains one or more metal atoms. (2) The water-soluble vitamins are the B vitamins and vitamins A and C. (3) An enzyme’s turnover number is the rate at which it is degraded and resynthesized within the human body. a) All three statem ...
Chapter 2 Biochemistry Goux Guided Notes
... - Catalysts work by lowering a reaction’s _____activation energy____ - ___Enzymes_____ are proteins that act as catalysts. - The name of an enzyme is derived from the reaction it __catalyzes__ - Enzymes names end with ___ -ase____ Enzymes speed up chemical reactions that take place in cells. - The s ...
... - Catalysts work by lowering a reaction’s _____activation energy____ - ___Enzymes_____ are proteins that act as catalysts. - The name of an enzyme is derived from the reaction it __catalyzes__ - Enzymes names end with ___ -ase____ Enzymes speed up chemical reactions that take place in cells. - The s ...
N -glutamate Iminohydrolase from Pseudomonas aeruginosa L
... wild type enzyme. However, the Michaelis constants were in an experimentally accessible range. pH-Rate Profiles. The kinetic constants for the enzymatic hydrolysis of N-formimino-L-glutamate by HutF were determined as a function of pH. The plots of kcat and kcat/Km versus pH for the zinc substituted ...
... wild type enzyme. However, the Michaelis constants were in an experimentally accessible range. pH-Rate Profiles. The kinetic constants for the enzymatic hydrolysis of N-formimino-L-glutamate by HutF were determined as a function of pH. The plots of kcat and kcat/Km versus pH for the zinc substituted ...
Unit 1.1 Building Blocks of Life The student knows the significance of
... The student knows the significance of various molecules involved in metabolic processes and energy conversions that occur in living organisms. The student is expected to: ...
... The student knows the significance of various molecules involved in metabolic processes and energy conversions that occur in living organisms. The student is expected to: ...
Document
... Increased solubility of the hydrolyzed protein is usually due to increase in the number of small peptides, and the corresponding increase in the ionizable amino and carboxyl groups Hydrolysis processes needs to be controlled to improve solubility Hydrolysates can expose hydrophobic peptides wh ...
... Increased solubility of the hydrolyzed protein is usually due to increase in the number of small peptides, and the corresponding increase in the ionizable amino and carboxyl groups Hydrolysis processes needs to be controlled to improve solubility Hydrolysates can expose hydrophobic peptides wh ...
Document
... Some proteins fairly rigid = “fixed” structure often known from crystallography Others don’t crystallize, probably “floppy” (or have parts that are floppy) in solution Some have a few, alternative “rigid” shapes (important!) Surface distribution of charged, polar (partially charged), hydrophobic, e ...
... Some proteins fairly rigid = “fixed” structure often known from crystallography Others don’t crystallize, probably “floppy” (or have parts that are floppy) in solution Some have a few, alternative “rigid” shapes (important!) Surface distribution of charged, polar (partially charged), hydrophobic, e ...
Macromolecule Basics
... • This is the most common organic molecule • It makes up most plant matter • They are made from carbon, hydrogen and oxygen • Their building block is a single sugar called a monosaccharide (mono = single) • When 2 sugars combine it is called a ...
... • This is the most common organic molecule • It makes up most plant matter • They are made from carbon, hydrogen and oxygen • Their building block is a single sugar called a monosaccharide (mono = single) • When 2 sugars combine it is called a ...
Three-Point Binding Model
... align three binding sites to give high specificity • One problem with model: – Model is a static representation → “lock & key” ...
... align three binding sites to give high specificity • One problem with model: – Model is a static representation → “lock & key” ...
THERMODYNAMICS AND ENZYMES STUDY GUIDE
... majority of organic molecules to make it over the hump of activation energy. ...
... majority of organic molecules to make it over the hump of activation energy. ...
Dark Reactions
... by ferredoxin-thioredoxin reductase. This enzyme has a 4Fe-4S cluster that couples the one electron transfers from reduced ferredoxin towards the two electron reduction of thioredoxin. Phosphoribulose kinase and glyceraldehydes 3phosphate dehydrogenase are also regulated by NADPH directly. In the da ...
... by ferredoxin-thioredoxin reductase. This enzyme has a 4Fe-4S cluster that couples the one electron transfers from reduced ferredoxin towards the two electron reduction of thioredoxin. Phosphoribulose kinase and glyceraldehydes 3phosphate dehydrogenase are also regulated by NADPH directly. In the da ...
the Four Stages of Biochemical Energy Production
... Stages 3 & 4 • These stages are the same for all types of food = “common metabolic pathway” • They occur inside the mitochondria – Citric Acid (Kreb’s) Cycle – Electron Transport Chain & Oxidative Phosphorylation ...
... Stages 3 & 4 • These stages are the same for all types of food = “common metabolic pathway” • They occur inside the mitochondria – Citric Acid (Kreb’s) Cycle – Electron Transport Chain & Oxidative Phosphorylation ...
free energy
... Substrate Specificity of Enzymes • The reactant that an enzyme acts on is called the enzyme’s substrate • The enzyme binds to its substrate, forming an enzyme-substrate complex • The active site is the region on the enzyme where the substrate binds • Induced fit of a substrate brings chemical group ...
... Substrate Specificity of Enzymes • The reactant that an enzyme acts on is called the enzyme’s substrate • The enzyme binds to its substrate, forming an enzyme-substrate complex • The active site is the region on the enzyme where the substrate binds • Induced fit of a substrate brings chemical group ...
Cell walls
... (matrix polysaccharides) present in almost all plant cell walls along with cellulose. Cellulose contains only anhydrous glucose, while hemicellulose contains many different sugar monomers. For instance, besides glucose, sugar monomers in hemicellulose can include xylose, mannose, galactose, rhamno ...
... (matrix polysaccharides) present in almost all plant cell walls along with cellulose. Cellulose contains only anhydrous glucose, while hemicellulose contains many different sugar monomers. For instance, besides glucose, sugar monomers in hemicellulose can include xylose, mannose, galactose, rhamno ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.