Lecture 19 TCA Cycle 1. How pyruvate is converted to acetyl
... 1. How pyruvate is converted to acetyl-CoA which is a precursor for TCA cycle? Answer: The pyruvic molecules formed in glycolosis enter the mitochondria, where they are converted to acetyl coenzyme A (acetyl CoA). In this complex series of reactions, pyruvate undergoes oxidative decarboxylation. Fir ...
... 1. How pyruvate is converted to acetyl-CoA which is a precursor for TCA cycle? Answer: The pyruvic molecules formed in glycolosis enter the mitochondria, where they are converted to acetyl coenzyme A (acetyl CoA). In this complex series of reactions, pyruvate undergoes oxidative decarboxylation. Fir ...
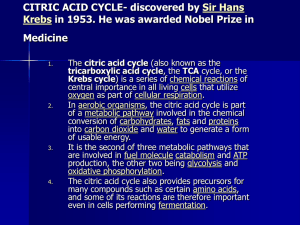
CITRIC ACID CYCLE
... It is the second of three metabolic pathways that are involved in fuel molecule catabolism and ATP production, the other two being glycolysis and oxidative phosphorylation. The citric acid cycle also provides precursors for many compounds such as certain amino acids, and some of its reactions are th ...
... It is the second of three metabolic pathways that are involved in fuel molecule catabolism and ATP production, the other two being glycolysis and oxidative phosphorylation. The citric acid cycle also provides precursors for many compounds such as certain amino acids, and some of its reactions are th ...
3.2 and 3.3
... Monomer of Nucleic acids are….. Name the three groups in one monomer… Nucleic acids primary function is to …… What process puts these monomers together to form long chains…. • What process breaks down ATP for energy….. ...
... Monomer of Nucleic acids are….. Name the three groups in one monomer… Nucleic acids primary function is to …… What process puts these monomers together to form long chains…. • What process breaks down ATP for energy….. ...
Final Key - UC Davis Plant Sciences
... glucose that can be used as a fuel by the other cells of the body. On the other hand, the skeletal muscles are specialized for energy production (ATP). Thus, if glycolysis is activated in the muscles (ATP production), gluconeogenesis will be activated in the liver to produce glucose for the muscles ...
... glucose that can be used as a fuel by the other cells of the body. On the other hand, the skeletal muscles are specialized for energy production (ATP). Thus, if glycolysis is activated in the muscles (ATP production), gluconeogenesis will be activated in the liver to produce glucose for the muscles ...
CH 8
... Enzymes use a variety of mechanisms to lower activation energy and speed up a reaction. In reactions involving more than one reactant, the active site brings substrates together in the correct orientation for the reaction to proceed. As the active site binds the substrate, it may put stress on b ...
... Enzymes use a variety of mechanisms to lower activation energy and speed up a reaction. In reactions involving more than one reactant, the active site brings substrates together in the correct orientation for the reaction to proceed. As the active site binds the substrate, it may put stress on b ...
BCHEM 253 – METABOLISM IN HEALTH AND DISEASES
... 2. The transfer of phosphoryl groups conserves metabolic energy. The energy released in breaking the phosphoanhydride bonds of ATP is partially conserved in the formation of phosphate esters. High-energy phosphate compounds formed in glycolysis donate phosphoryl groups to ADP to form ATP. 3. The en ...
... 2. The transfer of phosphoryl groups conserves metabolic energy. The energy released in breaking the phosphoanhydride bonds of ATP is partially conserved in the formation of phosphate esters. High-energy phosphate compounds formed in glycolysis donate phosphoryl groups to ADP to form ATP. 3. The en ...
Rat Leptin ELISA Kit wako
... Preparation: The enzyme is dissolved in 5 mM DTT, 50% glycerin and 50 mM Sodium phosphate buffer solution (pH 7.0). Aldose Reductase is purified from the culture medium containing baculovirus-insect cell line 32d gene expression system. cDNA containing the entire protein coding region is expressed, ...
... Preparation: The enzyme is dissolved in 5 mM DTT, 50% glycerin and 50 mM Sodium phosphate buffer solution (pH 7.0). Aldose Reductase is purified from the culture medium containing baculovirus-insect cell line 32d gene expression system. cDNA containing the entire protein coding region is expressed, ...
Biochemistry - Circle of Docs
... 79. if 14:2 fatty acid passes through beta oxidation the end result will be _______ and cleave _________ many times (stupid question) 80. involved in decarboxylation a. thiamine b. biotin c. pyridoxine d. riboflavin 81. cofactor for transamination a. pyridoxine 82. makes of CoA a. B5 b. B1 c. B6 d. ...
... 79. if 14:2 fatty acid passes through beta oxidation the end result will be _______ and cleave _________ many times (stupid question) 80. involved in decarboxylation a. thiamine b. biotin c. pyridoxine d. riboflavin 81. cofactor for transamination a. pyridoxine 82. makes of CoA a. B5 b. B1 c. B6 d. ...
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN: 2278-3008.
... change may be a result of changes in the activity of enzymes. The activity of plant polyphenoloxidases can cause enzymatic browning of fruits and vegetables and render food unfit for consumption due to formation of benzoquinone. Proteases degrade polypeptides and facilitate seed germination, leaf se ...
... change may be a result of changes in the activity of enzymes. The activity of plant polyphenoloxidases can cause enzymatic browning of fruits and vegetables and render food unfit for consumption due to formation of benzoquinone. Proteases degrade polypeptides and facilitate seed germination, leaf se ...
The Working Cell
... • 2. Enzymes speed chemical reactions by lowering the energy of activation (Ea) by forming a complex with their substrate(s) at the active site. • a. An active site is a small region on the surface of the enzyme where the substrate(s) bind. • b. When a substrate binds to an enzyme, the active site u ...
... • 2. Enzymes speed chemical reactions by lowering the energy of activation (Ea) by forming a complex with their substrate(s) at the active site. • a. An active site is a small region on the surface of the enzyme where the substrate(s) bind. • b. When a substrate binds to an enzyme, the active site u ...
AP Biology Question Set
... 48. The conversion of ATP to ADP and Pi releases approxi mately 7.3 kcal/mol of energy. This energy release fuels (endergonic) reactions in the cell. Equilibrium of the reaction is far to the right and favors the formation of ADP. In the converse, the formation of ATP from ADP and Pi is energy inten ...
... 48. The conversion of ATP to ADP and Pi releases approxi mately 7.3 kcal/mol of energy. This energy release fuels (endergonic) reactions in the cell. Equilibrium of the reaction is far to the right and favors the formation of ADP. In the converse, the formation of ATP from ADP and Pi is energy inten ...
Carboxypeptidase A - Chemistry Courses: About
... a resolution of 1.54 A,4 and additional X-ray experiments have identified four out of five subsites on the enzyme in the product complex with the 39 amino acid inhibitor from the pot at^.^ Recent high resolution crystallographic experiments have shown that typical substrate analogues,6 transition-st ...
... a resolution of 1.54 A,4 and additional X-ray experiments have identified four out of five subsites on the enzyme in the product complex with the 39 amino acid inhibitor from the pot at^.^ Recent high resolution crystallographic experiments have shown that typical substrate analogues,6 transition-st ...
Directed Enzyme Evolution and High
... do not fulfill the requirements of these harsh industrial conditions, and optimization is necessary to obtain a suitable enzyme catalyst for production needs. This tailoring of enzymes can be accomplished through two experimental routes. The first is rational design, which targets specific residues ...
... do not fulfill the requirements of these harsh industrial conditions, and optimization is necessary to obtain a suitable enzyme catalyst for production needs. This tailoring of enzymes can be accomplished through two experimental routes. The first is rational design, which targets specific residues ...
Apyrase from potato (A6237) - Datasheet - Sigma
... A large number of plant and animal tissues contain pyrophosphohydrolases commonly called apyrases. These enzymes catalyze the hydrolysis of a broad range of nucleoside tri- and di-phosphates.1,2 ATP → ADP + Pi → AMP + 2 Pi Some characteristics distinguish apyrases from other phosphohydrolases, such ...
... A large number of plant and animal tissues contain pyrophosphohydrolases commonly called apyrases. These enzymes catalyze the hydrolysis of a broad range of nucleoside tri- and di-phosphates.1,2 ATP → ADP + Pi → AMP + 2 Pi Some characteristics distinguish apyrases from other phosphohydrolases, such ...
Macromolecule Scramble
... o cells, tissue fluid, or in fluids being transported (blood or phloem) metabolic roles Ex: enzymes in all organisms, plasma proteins and antibodies in mammals Fibrous form long fibres mostly consist of repeated sequences of amino acids which are insoluble in water usually have structura ...
... o cells, tissue fluid, or in fluids being transported (blood or phloem) metabolic roles Ex: enzymes in all organisms, plasma proteins and antibodies in mammals Fibrous form long fibres mostly consist of repeated sequences of amino acids which are insoluble in water usually have structura ...
L11 Biochem alterations postharv storage - e
... contradicting because: First, cathepsins in intact muscle tissue are confined within the lysosomal membrane, i.e., they are not in direct contact with myofibrils. Second, these proteases have a very low pH requirement for optimal activity (1 to 2 pH units lower than postrigor meat pH). Third, electr ...
... contradicting because: First, cathepsins in intact muscle tissue are confined within the lysosomal membrane, i.e., they are not in direct contact with myofibrils. Second, these proteases have a very low pH requirement for optimal activity (1 to 2 pH units lower than postrigor meat pH). Third, electr ...
Molecules of Life
... 3. Detect environmental changes 4. Change rate of a reaction 5. Control genes 6. Cell communication 7. Trigger changes inside cell 8. Carry molecules from place to place 9. Stockpile building materials 10. Can move parts of a cell or animal ...
... 3. Detect environmental changes 4. Change rate of a reaction 5. Control genes 6. Cell communication 7. Trigger changes inside cell 8. Carry molecules from place to place 9. Stockpile building materials 10. Can move parts of a cell or animal ...
Recitation Notes for RDM Day 1 1. Module Overview –
... particular locations to generate specific DNA fragments. There are normally 4-5 components in a given reaction. 1- DNA which will be digested. Must have specific site for enzyme. Also needs to be free of contaminants such as phenol, alcohol, excessive salts, which interfere with enzyme activity. 2- ...
... particular locations to generate specific DNA fragments. There are normally 4-5 components in a given reaction. 1- DNA which will be digested. Must have specific site for enzyme. Also needs to be free of contaminants such as phenol, alcohol, excessive salts, which interfere with enzyme activity. 2- ...
08_LectureOutline_LO
... majority of organic molecules to make it over the hump of activation energy. ...
... majority of organic molecules to make it over the hump of activation energy. ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.