
BIOT 3 Lab 3 Handout 1
... defense mechanism to protect against infectious pathogens such as viruses called bacteriophage, also known as phage. Phage viruses reproduce by injecting DNA into a host bacteria and then use the host’s cellular machinery to replicate more copies of phage virus. Bacteria produce restriction enzymes ...
... defense mechanism to protect against infectious pathogens such as viruses called bacteriophage, also known as phage. Phage viruses reproduce by injecting DNA into a host bacteria and then use the host’s cellular machinery to replicate more copies of phage virus. Bacteria produce restriction enzymes ...
Modeling allosteric regulation of de novo pyrimidine biosynthesis in
... information to develop a new level of understanding of the metabolic complexity of the cell. The biosynthetic pathway of de novo pyrimidine nucleotide metabolism is an essential capability of all free-living cells, and it occupies a pivotal position relative to metabolic processes that are involved ...
... information to develop a new level of understanding of the metabolic complexity of the cell. The biosynthetic pathway of de novo pyrimidine nucleotide metabolism is an essential capability of all free-living cells, and it occupies a pivotal position relative to metabolic processes that are involved ...
Amino Acids, Proteins and Enzymes
... Shape-Determining Interactions in Proteins • H-bonding along backbone • R-group interactions – H-bonding – Hydrophobic interactions – Salt bridges – Covalent bonds • S-S ...
... Shape-Determining Interactions in Proteins • H-bonding along backbone • R-group interactions – H-bonding – Hydrophobic interactions – Salt bridges – Covalent bonds • S-S ...
Introduction
... Actuality of the theme. Amino acids are organic low-molecular compounds, which are included into the structure of proteins and peptides, and also are in the cells of organism in the free state and take part in metabolism. Physical and chemical properties of amino acids are predetermined by features ...
... Actuality of the theme. Amino acids are organic low-molecular compounds, which are included into the structure of proteins and peptides, and also are in the cells of organism in the free state and take part in metabolism. Physical and chemical properties of amino acids are predetermined by features ...
Handout
... wound healing to viral replication, proteases can be broadly lumped into two camps based on what’s in their ...
... wound healing to viral replication, proteases can be broadly lumped into two camps based on what’s in their ...
Reconstruction of Amino Acid Biosynthesis Pathways from the
... identified. Obviously, the functional prediction from a complete genome imposes an additional constraint of the completeness; for example, the total number of aminoacyl tRNA synthetases, the group of proteins that form an ABC transport system, and the group of enzymes that form a specific biosynthet ...
... identified. Obviously, the functional prediction from a complete genome imposes an additional constraint of the completeness; for example, the total number of aminoacyl tRNA synthetases, the group of proteins that form an ABC transport system, and the group of enzymes that form a specific biosynthet ...
Molecular and Structural Characterization of
... (Niemeyer, 1988). These compounds are thought to be stored in intact plants as glucosides within a different subcellular compartment from the glucosidase. Although the wheat and rye glucosidases hydrolyze DIBOA-Glc and DIMBOA-Glc, the preferred natural substrate for each enzyme is consistent with th ...
... (Niemeyer, 1988). These compounds are thought to be stored in intact plants as glucosides within a different subcellular compartment from the glucosidase. Although the wheat and rye glucosidases hydrolyze DIBOA-Glc and DIMBOA-Glc, the preferred natural substrate for each enzyme is consistent with th ...
fat-soluble vitamins
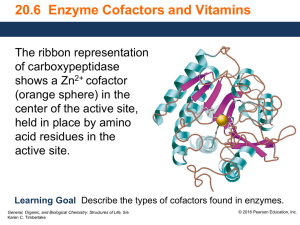
... Enzyme Cofactors • A simple enzyme is an active enzyme that consists only of protein. • Many enzymes are active only when they combine with cofactors such as metal ions or small molecules. • A coenzyme is a cofactor that is a small organic molecule such as a vitamin. Core Chemistry Skill Describing ...
... Enzyme Cofactors • A simple enzyme is an active enzyme that consists only of protein. • Many enzymes are active only when they combine with cofactors such as metal ions or small molecules. • A coenzyme is a cofactor that is a small organic molecule such as a vitamin. Core Chemistry Skill Describing ...
Amino Acids as Protein Building Blocks [2]
... primary sequence of amino acids. The physical chemical properties of the amino acids contain the biological information required for folding and function. ...
... primary sequence of amino acids. The physical chemical properties of the amino acids contain the biological information required for folding and function. ...
comparison free energy binding sites
... Table.1.comparison Potential energy (Kcal/mol) at 310 K in different dielectric ...
... Table.1.comparison Potential energy (Kcal/mol) at 310 K in different dielectric ...
The trimethoprim-resistant dihydrofolate reductase associated with
... Restriction enzyme analysis. DNA was isolated and cleaved with restriction enzymes as previously described (7) . The restriction nucleases BamHl, EcoRl, Sau3A and Haelll were kindly donated by T. Igo-Kemenes. Alul, Pstl, Hhal, Hinfl, Sau96l, and TagI were gifts of R.E. Streeck. Hinfl was prepared by ...
... Restriction enzyme analysis. DNA was isolated and cleaved with restriction enzymes as previously described (7) . The restriction nucleases BamHl, EcoRl, Sau3A and Haelll were kindly donated by T. Igo-Kemenes. Alul, Pstl, Hhal, Hinfl, Sau96l, and TagI were gifts of R.E. Streeck. Hinfl was prepared by ...
biochemistry module elective course contents
... No prerequisites Water; sorpsion phenomena, types of water, water activity and food spoilage, water binding of meet. Lipids; component fatty acids, component glycerides, interesterification, phospholipids, unsaponifiables, autoxidation, hated fats, flavor reversion, hydrogenation. Proteins; amino ac ...
... No prerequisites Water; sorpsion phenomena, types of water, water activity and food spoilage, water binding of meet. Lipids; component fatty acids, component glycerides, interesterification, phospholipids, unsaponifiables, autoxidation, hated fats, flavor reversion, hydrogenation. Proteins; amino ac ...
MDH-GOT enzyme assay. - G-global www.group
... stages, except that at milky stages the activities were increased and its activity in nonirrigated seeds has been shown to be double that in irrigated seeds, which means the enzyme complex plays an important role during developing and formation of wheat seeds under drought stress. ...
... stages, except that at milky stages the activities were increased and its activity in nonirrigated seeds has been shown to be double that in irrigated seeds, which means the enzyme complex plays an important role during developing and formation of wheat seeds under drought stress. ...
PDF
... proposed for ketosteroid isomerase and other enzymes that active site hydrogen bonding groups provide energetic stabilization via “short, strong” or “low-barrier” hydrogen bonds that are formed due to matching of their pKa or proton affinity to that of the transition state. It has also been proposed t ...
... proposed for ketosteroid isomerase and other enzymes that active site hydrogen bonding groups provide energetic stabilization via “short, strong” or “low-barrier” hydrogen bonds that are formed due to matching of their pKa or proton affinity to that of the transition state. It has also been proposed t ...
Quiz 2 Review Sheet
... 8. How does a food calorie compare to an actual calorie of energy? 9. Describe the structure and function of both NAD+ and FAD. 10. Compare oxidation to reduction. In class we said you could never have one without the other and hence the term redox reaction. Explain why this is? 11. Write out the o ...
... 8. How does a food calorie compare to an actual calorie of energy? 9. Describe the structure and function of both NAD+ and FAD. 10. Compare oxidation to reduction. In class we said you could never have one without the other and hence the term redox reaction. Explain why this is? 11. Write out the o ...
CH524: bioinorganic chemistry
... •A specific biochemical function (structural or catalytic or regulatory type) should be associated with that particular element •Physiological Physiological deficiency appears when the element is removed from a purified diet •The deficiency can be relieved by the addition of that specific element ...
... •A specific biochemical function (structural or catalytic or regulatory type) should be associated with that particular element •Physiological Physiological deficiency appears when the element is removed from a purified diet •The deficiency can be relieved by the addition of that specific element ...
1 From Chemical Invariance to Genetic Variability - Wiley-VCH
... Retrodicting the Elements of Life ...
... Retrodicting the Elements of Life ...
acid
... multi enzyme complex consist of three enzymes. . The conversion of pyruvic acid to acetyl COA involves 5 types of reaction and each reaction is catalysed by different enzyme system these enzymes act as a multi enzyme system (complex).. ...
... multi enzyme complex consist of three enzymes. . The conversion of pyruvic acid to acetyl COA involves 5 types of reaction and each reaction is catalysed by different enzyme system these enzymes act as a multi enzyme system (complex).. ...
McDougall, K. J. and V. W. Woodword. Suppression
... vitro arportic tranrcarbomylore (ATCore) activity. (The pyr-3 mutants used here are denoted by the KS-prefix. KS16 onT KS20 ore AT&se+; KS23 and KS43 are AT&se‘. The arg~tontr ore designated CIS 6-l. 6-2, 6-3, 6-8 and 7.0.) The mechanism of suppression is thought to be due to metabolic crorr-feeding ...
... vitro arportic tranrcarbomylore (ATCore) activity. (The pyr-3 mutants used here are denoted by the KS-prefix. KS16 onT KS20 ore AT&se+; KS23 and KS43 are AT&se‘. The arg~tontr ore designated CIS 6-l. 6-2, 6-3, 6-8 and 7.0.) The mechanism of suppression is thought to be due to metabolic crorr-feeding ...
Chapter 5 Notes (Biomolecules)
... • Many chemical reactions occur inside cells Reactants products • Enzymes are protein catalysts that speed up reactions by lowering the activation energy needed to start a reaction. • Activation Energy -The energy needed to start up a reaction • Catalyst – any compound that speeds up a reaction by ...
... • Many chemical reactions occur inside cells Reactants products • Enzymes are protein catalysts that speed up reactions by lowering the activation energy needed to start a reaction. • Activation Energy -The energy needed to start up a reaction • Catalyst – any compound that speeds up a reaction by ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.







![Amino Acids as Protein Building Blocks [2]](http://s1.studyres.com/store/data/001231379_1-1c71e58dd2b4a4d156753bc870eff68b-300x300.png)














