The Mechanism of Propionic Acid Formation by
... the same redox level this difference in propionic :acetic ratio cannot be explained by the difference in the amounts of acetate converted to CO, due to their different levels of oxidation as indicated by the experiments of Carson (1948). It appears that the large variations reported in the literatur ...
... the same redox level this difference in propionic :acetic ratio cannot be explained by the difference in the amounts of acetate converted to CO, due to their different levels of oxidation as indicated by the experiments of Carson (1948). It appears that the large variations reported in the literatur ...
Biochemistry of Ensiling - DigitalCommons@University of Nebraska
... Two or more (up to 10) monosaccharides linked together are referred to as oligosaccharides (Kandler & Hopf, 1980). This is a bit of an arbitrary definition to draw a distinction between oligosaccharides and polysaccharides. In most cases naturally occurring oligosaccharides that are not intermediate ...
... Two or more (up to 10) monosaccharides linked together are referred to as oligosaccharides (Kandler & Hopf, 1980). This is a bit of an arbitrary definition to draw a distinction between oligosaccharides and polysaccharides. In most cases naturally occurring oligosaccharides that are not intermediate ...
Wk12 Acid base_lec
... • Interact extensively with other buffer systems 2. Carbonic acid–bicarbonate buffer system • Most important in ECF 3. Phosphate buffer system • Buffers pH of ICF and urine © 2012 Pearson Education, Inc. ...
... • Interact extensively with other buffer systems 2. Carbonic acid–bicarbonate buffer system • Most important in ECF 3. Phosphate buffer system • Buffers pH of ICF and urine © 2012 Pearson Education, Inc. ...
04 Purine_degradation-Gout
... The ingested bases are mostly degraded into different products by degradation pathways These products are then excreted by the body ...
... The ingested bases are mostly degraded into different products by degradation pathways These products are then excreted by the body ...
Introduction to Carbohydrates
... The first step in the catabolism of most amino acids is the transfer of their α-amino group to α-ketoglutarate (Figure 19.7). The products are an α-keto acid (derived from the original amino acid) and glutamate. α-Ketoglutarate plays a pivotal role in amino acid metabolism by accepting the ami ...
... The first step in the catabolism of most amino acids is the transfer of their α-amino group to α-ketoglutarate (Figure 19.7). The products are an α-keto acid (derived from the original amino acid) and glutamate. α-Ketoglutarate plays a pivotal role in amino acid metabolism by accepting the ami ...
Primary structure: the sequence of amino acids in a polypeptide chain
... Catalysts: virtually all reactions in living systems are catalyzed by proteins called enzymes Movement: muscles are made up of proteins called myosin and actin Transport: hemoglobin transports oxygen from the lungs to cells; other proteins transport molecules across cell membranes Hormones: many hor ...
... Catalysts: virtually all reactions in living systems are catalyzed by proteins called enzymes Movement: muscles are made up of proteins called myosin and actin Transport: hemoglobin transports oxygen from the lungs to cells; other proteins transport molecules across cell membranes Hormones: many hor ...
ConcepTest On Simple Redox Reactions
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
Metabolic Integration during the Postprandial, Fasting and Feedback
... the mobilization of this carrier from the storage sites and their migration to the membrane plasma [3,4,7,19]. Another action of insulin in muscle tissue is the inhibition of protein degradation with favor of protein synthesis [4,6,20,21], thereby, adequate diets in amino acids and carbohydrates be ...
... the mobilization of this carrier from the storage sites and their migration to the membrane plasma [3,4,7,19]. Another action of insulin in muscle tissue is the inhibition of protein degradation with favor of protein synthesis [4,6,20,21], thereby, adequate diets in amino acids and carbohydrates be ...
Cells and Energy
... Photosynthesis is a process that captures energy from sunlight to make sugars that store chemical energy. Therefore, directly or indirectly, the energy for almost all organisms begins as sunlight. Sunlight includes a wide range of radiant energy, such as ultraviolet radiation, microwaves, and the ...
... Photosynthesis is a process that captures energy from sunlight to make sugars that store chemical energy. Therefore, directly or indirectly, the energy for almost all organisms begins as sunlight. Sunlight includes a wide range of radiant energy, such as ultraviolet radiation, microwaves, and the ...
Protein Synthesis I
... iii. Only about ½ of the proteins we make get folded- one reason is that there is a 10-20% failure of synthetases to put the right amino acid on the right tRNA 1. So not all proteins are synthesized properly 2. That is one reason why it is more economical to make quaternary structures out of small n ...
... iii. Only about ½ of the proteins we make get folded- one reason is that there is a 10-20% failure of synthetases to put the right amino acid on the right tRNA 1. So not all proteins are synthesized properly 2. That is one reason why it is more economical to make quaternary structures out of small n ...
Protein Catabolism
... The biosynthesis of proteins requires a continuous source of amino acids. Amino acids also provide cells with a source of nitrogen for the synthesis of nitrogen containing biomolecules such as nucleotides. Amino acids are generated by the digestion of proteins in the intestine or by the degradation ...
... The biosynthesis of proteins requires a continuous source of amino acids. Amino acids also provide cells with a source of nitrogen for the synthesis of nitrogen containing biomolecules such as nucleotides. Amino acids are generated by the digestion of proteins in the intestine or by the degradation ...
Chymotrypsin is a Serine Protease
... Proton donors can also catalyze reactions • A general acid (BH+) can donate protons • A covalent bond may break more easily if one of its atoms is protonated (below) ...
... Proton donors can also catalyze reactions • A general acid (BH+) can donate protons • A covalent bond may break more easily if one of its atoms is protonated (below) ...
D-lactic acidosis: Turning sugar into acids in the gastrointestinal tract
... because organic acids accumulated within the GI tract, one can renal failure, the degree of organic acid accumulation could be infer that bacterial overgrowth may still be a potential hazard; a much greater for a given rate of production in the GI tract. To analyze the acid-base consequences from a ...
... because organic acids accumulated within the GI tract, one can renal failure, the degree of organic acid accumulation could be infer that bacterial overgrowth may still be a potential hazard; a much greater for a given rate of production in the GI tract. To analyze the acid-base consequences from a ...
Glucose metabolism in Trypanosoma cruzi
... ylation sites 2 and 3 are operative; the presence of a NADH oxidase linked to phosphorylation (complex I) has been long debated. Recently, Carranza et al. [28] have compared oxygen consumption and respiratory control ratios in the presence of NADH‑linked substrates or succinate in wild‑type T. cruzi ...
... ylation sites 2 and 3 are operative; the presence of a NADH oxidase linked to phosphorylation (complex I) has been long debated. Recently, Carranza et al. [28] have compared oxygen consumption and respiratory control ratios in the presence of NADH‑linked substrates or succinate in wild‑type T. cruzi ...
Cellular Respiration Webquest
... (http://www.biologyinmotion.com/atp/index.html) 1. What is ATP? ...
... (http://www.biologyinmotion.com/atp/index.html) 1. What is ATP? ...
GI Digest - Douglas Labs
... proteins, and fats, so that the breakdown products can be absorbed in the upper small intestine. Amylase is the major carbohydrate-digesting enzyme. Lipases break down triglycerides into monoglycerides and free fatty acids, which are efficiently absorbed in the upper small intestine. Protein digesti ...
... proteins, and fats, so that the breakdown products can be absorbed in the upper small intestine. Amylase is the major carbohydrate-digesting enzyme. Lipases break down triglycerides into monoglycerides and free fatty acids, which are efficiently absorbed in the upper small intestine. Protein digesti ...
Determination of Fatty Acids and Carbohydrate Monomers in Micro
... somewhat larger variations. No differences could be observed between chromatograms representing preparations derivatized 1 week or 1 year after the mycobacteria had been lyophilized. Derivatized samples could be stored for at least 1 week at - 20 "C without any change in their chromatographic patter ...
... somewhat larger variations. No differences could be observed between chromatograms representing preparations derivatized 1 week or 1 year after the mycobacteria had been lyophilized. Derivatized samples could be stored for at least 1 week at - 20 "C without any change in their chromatographic patter ...
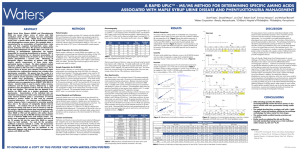
A Rapid UPLC™ MS/MS Method for Determining Specific
... metabolism which, if left untreated, can have catastrophic consequences for the child. Maple Syrup Urine Disease results from a genetic defect of the branched-chain a-keto acid dehydrogenase enzyme system. This metabolic defect is characterized by an accumulation of branched-chain α-keto acids and t ...
... metabolism which, if left untreated, can have catastrophic consequences for the child. Maple Syrup Urine Disease results from a genetic defect of the branched-chain a-keto acid dehydrogenase enzyme system. This metabolic defect is characterized by an accumulation of branched-chain α-keto acids and t ...
Determination of Fatty Acids and Carbohydrate Monomers in Micro
... somewhat larger variations. No differences could be observed between chromatograms representing preparations derivatized 1 week or 1 year after the mycobacteria had been lyophilized. Derivatized samples could be stored for at least 1 week at - 20 "C without any change in their chromatographic patter ...
... somewhat larger variations. No differences could be observed between chromatograms representing preparations derivatized 1 week or 1 year after the mycobacteria had been lyophilized. Derivatized samples could be stored for at least 1 week at - 20 "C without any change in their chromatographic patter ...
Principles of Metabolic Regulation
... require energy for extended periods of time. For example, ducks generally fly several thousand miles during their annual migration. The flight muscles of migratory birds have a high oxidative capacity and obtain the necessary ATP through the oxidation of acetyl-CoA (obtained from fats) via the citri ...
... require energy for extended periods of time. For example, ducks generally fly several thousand miles during their annual migration. The flight muscles of migratory birds have a high oxidative capacity and obtain the necessary ATP through the oxidation of acetyl-CoA (obtained from fats) via the citri ...
Full_ppt_ch20
... pH and Ionization • Acidic amino acids such as aspartic acid have a second carboxyl group that can donate and accept protons – Amino acids with ionizable side chains have 4 forms in solution • -Cys, Tyr, Lys, Arg, His, Asp, Glu ...
... pH and Ionization • Acidic amino acids such as aspartic acid have a second carboxyl group that can donate and accept protons – Amino acids with ionizable side chains have 4 forms in solution • -Cys, Tyr, Lys, Arg, His, Asp, Glu ...
Carbon conversion efficiency and central - Shachar
... reducing power in the form of NADH and NADPH. In green seeds, light energy can be used by chloroplasts to provide the ATP and NADPH necessary for fatty acid synthesis (Browse and Slack, 1985; Goffman et al., 2005; Ruuska et al., 2004; Schwender et al., 2004a, 2006), whereas plastids isolated from he ...
... reducing power in the form of NADH and NADPH. In green seeds, light energy can be used by chloroplasts to provide the ATP and NADPH necessary for fatty acid synthesis (Browse and Slack, 1985; Goffman et al., 2005; Ruuska et al., 2004; Schwender et al., 2004a, 2006), whereas plastids isolated from he ...
A1986A459700001
... production of a-amylase by de-embryonated grain as a basis for a bioassay for gibberellin. My experiments showed that the synthesis of a-amylase in half-grains of barley was proportional to the logarithm of the gibberellie acid concentration and could be detected at hormone concentrations as low as ...
... production of a-amylase by de-embryonated grain as a basis for a bioassay for gibberellin. My experiments showed that the synthesis of a-amylase in half-grains of barley was proportional to the logarithm of the gibberellie acid concentration and could be detected at hormone concentrations as low as ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.