The First, Second, and Third Law of Thermodynamics (ThLaws05.tex)
... The laws of thermodynamics apply to well-de…ned systems. First we will discuss a quite general form of the …rst and second law. I.e. we consider a system which is inhomogeneous, we allow mass transfer across the boundaries (open system), and we allow the boundaries to move. Fig.1 is a general repres ...
... The laws of thermodynamics apply to well-de…ned systems. First we will discuss a quite general form of the …rst and second law. I.e. we consider a system which is inhomogeneous, we allow mass transfer across the boundaries (open system), and we allow the boundaries to move. Fig.1 is a general repres ...
Practice Problems and Solutions for Quiz: 100g of water was
... Practice Problems and Solutions for Quiz: 1. 100g of water was warmed 50 degrees C. Find the energy in Joules and calories. ...
... Practice Problems and Solutions for Quiz: 1. 100g of water was warmed 50 degrees C. Find the energy in Joules and calories. ...
Solutions - University of Illinois at Chicago
... A vertical cylinder contains n moles of an ideal gas, and is closed off by a piston of mass M and area A. The acceleration due to gravity is g. The molar specific heat Cv (at constant volume) of the gas is a constant independent of temperature. The heat capacities of the piston and the cylinder are ...
... A vertical cylinder contains n moles of an ideal gas, and is closed off by a piston of mass M and area A. The acceleration due to gravity is g. The molar specific heat Cv (at constant volume) of the gas is a constant independent of temperature. The heat capacities of the piston and the cylinder are ...
Current Winter Processes Modeling Approaches
... Winter Routines are active when: • A snowpack already exists, or • A soil frost layer already exists, or • Average daily temperature is less than 0oC ...
... Winter Routines are active when: • A snowpack already exists, or • A soil frost layer already exists, or • Average daily temperature is less than 0oC ...
0 Quarter Three Assessment Review - SRHSchem
... the solid is completely dissolve, the water temperature drops to 15.2°C. a. Is this process endothermic or exothermic? Explain. – The temperature of the water decreases, so the dissolving process must have absorbed that heat, ...
... the solid is completely dissolve, the water temperature drops to 15.2°C. a. Is this process endothermic or exothermic? Explain. – The temperature of the water decreases, so the dissolving process must have absorbed that heat, ...
homeostasis - advbiology227
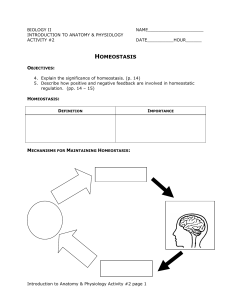
... In general, what effect does negative feedback have on homeostasis? _____________________________________________________________ In general, what effect does positive feedback have on homeostasis? _____________________________________________________________ ...
... In general, what effect does negative feedback have on homeostasis? _____________________________________________________________ In general, what effect does positive feedback have on homeostasis? _____________________________________________________________ ...
Document
... The first term is positive and the second is negative. After a complete cycle, the system is back to its original state, which means it is back to its original pressure, volume, temperature, internal energy, and entropy. Thus, S for a complete cycle must be zero. ...
... The first term is positive and the second is negative. After a complete cycle, the system is back to its original state, which means it is back to its original pressure, volume, temperature, internal energy, and entropy. Thus, S for a complete cycle must be zero. ...
PHYSICS 1030L LAB: Heat of Fusion
... to condense on the outer surface, how would this affect the final temperature? 3. Knowing the uncertainties in all your measurements, which variable will cause the largest difference between the Theoretical value of the Latent Heat of Fusion and your experimental value? 4. In our experiment we took i ...
... to condense on the outer surface, how would this affect the final temperature? 3. Knowing the uncertainties in all your measurements, which variable will cause the largest difference between the Theoretical value of the Latent Heat of Fusion and your experimental value? 4. In our experiment we took i ...
Course 3: Pressure – Volume – Temperature Relationship of Pure
... The P-V-T relationship of fluids is hard to formulate However relatively simple equations can Describe the P-V-T behavior of gas regions For gases P decreases proportionally to the increase in V Thus ...
... The P-V-T relationship of fluids is hard to formulate However relatively simple equations can Describe the P-V-T behavior of gas regions For gases P decreases proportionally to the increase in V Thus ...
Heat is energy transferring in a system and its surroundings.
... The latent heat is the energy involved in the change of phase for a material. The heat required to change a material from the liquid to solid state (or solid to liquid state) is called the heat of fusion. The heat required to change a material from the liquid to gas state (or gas to liquid state) is ...
... The latent heat is the energy involved in the change of phase for a material. The heat required to change a material from the liquid to solid state (or solid to liquid state) is called the heat of fusion. The heat required to change a material from the liquid to gas state (or gas to liquid state) is ...
Heat stress round table
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
Name: SOLUTIONS Physics 240, Exam #1 Sept. 24 2015 (4:15
... helium does during the expansion. If the water were frozen, it would not allow that expansion. Then the same temperature increase of 20.0 C° would require only 1250 J of energy, because no work would be done by the helium. If the surrounding water has nowhere to expand (like ice example above, or ve ...
... helium does during the expansion. If the water were frozen, it would not allow that expansion. Then the same temperature increase of 20.0 C° would require only 1250 J of energy, because no work would be done by the helium. If the surrounding water has nowhere to expand (like ice example above, or ve ...
chapter 13 (Homework) - Tutor
... (1) Type your answer for physical states using the format of g for gas, l for liquid, and s for solid. If more than one physical state is present, enter the physical states separated by a comma. (2) Type your answer for trends using the format of increase, decrease, remains constant. (3) Type your ...
... (1) Type your answer for physical states using the format of g for gas, l for liquid, and s for solid. If more than one physical state is present, enter the physical states separated by a comma. (2) Type your answer for trends using the format of increase, decrease, remains constant. (3) Type your ...
MME 2006 Metallurgical Thermodynamics
... Volumetric data of substances are needed to calculate the thermodynamic properties such as internal energy and enthalpy, from which the heat and work requirements of processes are obtained Understanding the P-V-T behavior of pure substances allows the engineer to make accurate estimations to the ch ...
... Volumetric data of substances are needed to calculate the thermodynamic properties such as internal energy and enthalpy, from which the heat and work requirements of processes are obtained Understanding the P-V-T behavior of pure substances allows the engineer to make accurate estimations to the ch ...