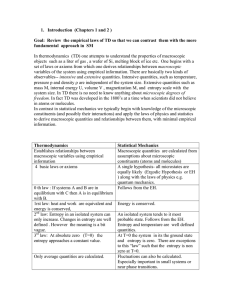
Practice Problems and Solutions for Quiz: 100g of water was
... 70g of water at 10C. Find the final temperature. gCpT= gCpT (80g)(0.9J/gC)(75-x)=(70g)(4.18J/gC)(x-10) 5,400-72x = 292.6x-2,926 8326=364.6x 22.84C = x ...
... 70g of water at 10C. Find the final temperature. gCpT= gCpT (80g)(0.9J/gC)(75-x)=(70g)(4.18J/gC)(x-10) 5,400-72x = 292.6x-2,926 8326=364.6x 22.84C = x ...
McMurry University Pre-test Practice Exam 1. Simplify each
... 23. The graph of a function is given. Use the graph to estimate the following. ...
... 23. The graph of a function is given. Use the graph to estimate the following. ...
Heat Heat Capacity Latent Heat Latent Heat
... This is electromagnetic radiation; objects at any temperature will emit it at various frequencies, from radio waves all the way to gamma rays. This radiation from a body in thermal equilibrium is called blackbody radiation, as it is purely thermal and doesn’t depend on any properties of the body oth ...
... This is electromagnetic radiation; objects at any temperature will emit it at various frequencies, from radio waves all the way to gamma rays. This radiation from a body in thermal equilibrium is called blackbody radiation, as it is purely thermal and doesn’t depend on any properties of the body oth ...
First Law of Thermodynamics
... Introduced the idea of internal energy. All of the energy of the system. Discussed that the internal energy is a state function. That is that it only depends on the state of the system (its particular properties T,V,P, ...) not how the system arrived in this state. Changes in internal energy are the ...
... Introduced the idea of internal energy. All of the energy of the system. Discussed that the internal energy is a state function. That is that it only depends on the state of the system (its particular properties T,V,P, ...) not how the system arrived in this state. Changes in internal energy are the ...
The Islamic University of Gaza
... 3- When a 25.0-g block of aluminum absorbs 10.0 kJ of heat, its temperature will rise how many degrees? The specific heat of Al is 0.900 J/ºC.g a. 444oC ...
... 3- When a 25.0-g block of aluminum absorbs 10.0 kJ of heat, its temperature will rise how many degrees? The specific heat of Al is 0.900 J/ºC.g a. 444oC ...
Algebra 1: Test #3 --- REVIEW---3 Show needed work, and write
... 36. When written in standard form, what is the constant of this equation? –8x2 + 6 = – 8x 37. Find the vertex of 38. What is the next step for solving this equation by ...
... 36. When written in standard form, what is the constant of this equation? –8x2 + 6 = – 8x 37. Find the vertex of 38. What is the next step for solving this equation by ...
More Than You Ever Cared to Know About Solution Thermodynamics
... The one dimensional hard line model, originally introduced by Rayleigh [1] and extended to hard line mixtures [2], was used to explicitly discuss solution thermodynamics [3]. Unfortunately, the hard line mixture (suitably extended to three dimensions) is an ideal solution, and as a result, part of t ...
... The one dimensional hard line model, originally introduced by Rayleigh [1] and extended to hard line mixtures [2], was used to explicitly discuss solution thermodynamics [3]. Unfortunately, the hard line mixture (suitably extended to three dimensions) is an ideal solution, and as a result, part of t ...
Comparison of Heat Loss by Sample Building Component
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
June - Westlands School Homework
... A booklet ‘Mathematical Formulae and Statistical Tables’ is provided. Full marks may be obtained for answers to ALL questions. There are 8 questions in this question paper. The total mark for this paper is 75. Advice to Candidates You must ensure that your answers to parts of questions are clearly l ...
... A booklet ‘Mathematical Formulae and Statistical Tables’ is provided. Full marks may be obtained for answers to ALL questions. There are 8 questions in this question paper. The total mark for this paper is 75. Advice to Candidates You must ensure that your answers to parts of questions are clearly l ...
Chemical Reactions (Chapters 7 and 8) Vocab and Reading
... 3. Balance each of the equations from question #2 (re-write each equation!). 4. What is the difference between a coefficient and a subscript in a chemical equation? Which can be changed when balancing a chemical equation? 5. What is the difference between an aqueous solution and a liquid? Chapter 8 ...
... 3. Balance each of the equations from question #2 (re-write each equation!). 4. What is the difference between a coefficient and a subscript in a chemical equation? Which can be changed when balancing a chemical equation? 5. What is the difference between an aqueous solution and a liquid? Chapter 8 ...
Thermochemistry
... • Heat energy can be used to not only change the temperature of matter, but also its phase. • The energy goes into separating or organizing the molecules into a new state • The amount of heat energy necessary to cause a phase change can be calculated using the formula: ...
... • Heat energy can be used to not only change the temperature of matter, but also its phase. • The energy goes into separating or organizing the molecules into a new state • The amount of heat energy necessary to cause a phase change can be calculated using the formula: ...
18493 Demonstrate knowledge of heat transfer in a seafood
... evidence is required for two factors for two of the following seafood processing operations – chilling, freezing, thawing, cooking, heat shocking, infra-red heat treatment, drying. ...
... evidence is required for two factors for two of the following seafood processing operations – chilling, freezing, thawing, cooking, heat shocking, infra-red heat treatment, drying. ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.