The Use and Misuse of the LUWS of Thermodynamics
... like Euclidean geometry from a small number of axioms which can he expressed in words, though much progress has been made toward the axiomatization of thermodynamic^,^ the mathematical structure has still not been completely separated from the physical content. But none of that need concern the unde ...
... like Euclidean geometry from a small number of axioms which can he expressed in words, though much progress has been made toward the axiomatization of thermodynamic^,^ the mathematical structure has still not been completely separated from the physical content. But none of that need concern the unde ...
What you absolutely have to know about Thermodynamics to pass
... energy as it moves from the hot place to the cold place. This stolen energy can be used to do useful work like generating electricity or moving our car down the street. The problem is that we can only siphon off energy while heat is being transferred. Once the hot place cools off the heat flow stops ...
... energy as it moves from the hot place to the cold place. This stolen energy can be used to do useful work like generating electricity or moving our car down the street. The problem is that we can only siphon off energy while heat is being transferred. Once the hot place cools off the heat flow stops ...
Heat Pumps and Refrigerators
... When a real heat engine is run backward, some of the intended work input (W ) goes into heat transfer before it gets into the heat engine, thereby reducing its coe cient of performance COPhp . In this gure, W ' represents the portion of W that goes into the heat pump, while the remainder of W is lo ...
... When a real heat engine is run backward, some of the intended work input (W ) goes into heat transfer before it gets into the heat engine, thereby reducing its coe cient of performance COPhp . In this gure, W ' represents the portion of W that goes into the heat pump, while the remainder of W is lo ...
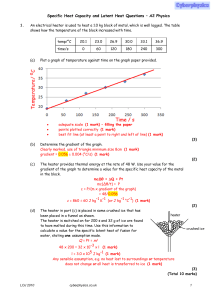
Specific Heat Capacity and Latent Heat Questions
... Ep = mgh = 0.025 × 9.81 × 1.2 = 0.29(4) J (1 mark) the total change in potential energy after 50 inversions, total change of energy (= 50 × 0.294) = 15 J (1 mark) (14.7 J) (use of 0.29 gives 14.(5) J) the specific heat capacity of the lead. ΔQ = mcΔθ 14.7 = 0.025 × c × 4.5 (1 mark) c = 131 J kg−1 K ...
... Ep = mgh = 0.025 × 9.81 × 1.2 = 0.29(4) J (1 mark) the total change in potential energy after 50 inversions, total change of energy (= 50 × 0.294) = 15 J (1 mark) (14.7 J) (use of 0.29 gives 14.(5) J) the specific heat capacity of the lead. ΔQ = mcΔθ 14.7 = 0.025 × c × 4.5 (1 mark) c = 131 J kg−1 K ...
Your Paper`s Title Starts Here: Please Center
... The cookoff of explosives refers to the process of explosion of explosive materials due to local or bulk heating of explosives under the external heating stimuli such as fire or high-temperature surrounding. Studies on the occurrence of cookoff explosion as well as its violence are of particular imp ...
... The cookoff of explosives refers to the process of explosion of explosive materials due to local or bulk heating of explosives under the external heating stimuli such as fire or high-temperature surrounding. Studies on the occurrence of cookoff explosion as well as its violence are of particular imp ...
Thermochemistry (Ch 8)
... Thermochemistry: the study of energy (in the form of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy in the form of heat; show a positive value for quantity of heat (q > 0) exothermic reactions: release energ ...
... Thermochemistry: the study of energy (in the form of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy in the form of heat; show a positive value for quantity of heat (q > 0) exothermic reactions: release energ ...
Experiment 1
... “obviously bad” points may be ignored in the plots, but may not be omitted from the data. Any omission of data should be discussed in the report. Derived data: Present your data for each solution in Table 2 : molality, square root of molality, freezing point depression, and values for the osmotic co ...
... “obviously bad” points may be ignored in the plots, but may not be omitted from the data. Any omission of data should be discussed in the report. Derived data: Present your data for each solution in Table 2 : molality, square root of molality, freezing point depression, and values for the osmotic co ...
electronic transport in liquid lithium-lead alloys
... Abstract.- The self-consistent pseudopotential theory, developed previously for the binary alloys of simple metals, is applied to calculate (1) the excess electronic charges on the electronegative ions, i.e. Pb ions, due to the partial localization of the valence electrons on these ions and (2) the ...
... Abstract.- The self-consistent pseudopotential theory, developed previously for the binary alloys of simple metals, is applied to calculate (1) the excess electronic charges on the electronegative ions, i.e. Pb ions, due to the partial localization of the valence electrons on these ions and (2) the ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.