Chapter 1: Introductory Concepts, Units, and Definitions
... Notice from the steam property tables that we have also included three new properties: internal energy u [kJ/kg], enthalpy h [kJ/kg], and entropy s [kJ/kg.K] all of which will be defined as needed in future sections. At this stage we note that the 3 equations relating quality and specific volume ca ...
... Notice from the steam property tables that we have also included three new properties: internal energy u [kJ/kg], enthalpy h [kJ/kg], and entropy s [kJ/kg.K] all of which will be defined as needed in future sections. At this stage we note that the 3 equations relating quality and specific volume ca ...
lec13-problems-solutions
... • Air at a temperature of 15oC passes through a heat exchanger at a velocity of 30 m/s where its temperature is raised to 800oC. It then passes through a turbine with the same velocity of 30 m/s and expands until the temperature falls to 650oC. On leaving the turbine, the air is taken at a velocity ...
... • Air at a temperature of 15oC passes through a heat exchanger at a velocity of 30 m/s where its temperature is raised to 800oC. It then passes through a turbine with the same velocity of 30 m/s and expands until the temperature falls to 650oC. On leaving the turbine, the air is taken at a velocity ...
Development of a Prototype Thermal Management
... proposed using solid heat spreaders of high thermal conductivity interleaved between the chip layers. The spreaders conduct heat to the base of an advanced synthetic jet cooled heat sink. A prior computational study [1] showed that for moderate power dissipations, 5 W in each 27 × 38 mm layer, a 250 ...
... proposed using solid heat spreaders of high thermal conductivity interleaved between the chip layers. The spreaders conduct heat to the base of an advanced synthetic jet cooled heat sink. A prior computational study [1] showed that for moderate power dissipations, 5 W in each 27 × 38 mm layer, a 250 ...
Document
... 20-2 Heat Engines We will discuss only engines that run in a repeating cycle; the change in internal energy over a cycle is zero, as the system returns to its initial state. The high-temperature reservoir transfers an amount of heat QH to the engine, where part of it is transformed into work W and ...
... 20-2 Heat Engines We will discuss only engines that run in a repeating cycle; the change in internal energy over a cycle is zero, as the system returns to its initial state. The high-temperature reservoir transfers an amount of heat QH to the engine, where part of it is transformed into work W and ...
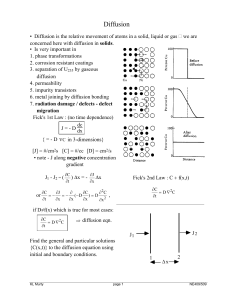
Diffusion
... Recalling Fick's first law {J = - D dx }, D = 2 α2 Γ or D = 6 α2 Γ This equation is similar to the one derived in the text using random-walk theory, ...
... Recalling Fick's first law {J = - D dx }, D = 2 α2 Γ or D = 6 α2 Γ This equation is similar to the one derived in the text using random-walk theory, ...
POTD-10-30
... Multiplying al l three numbers by 3 give the empirical formula C3H4O3, which has an empirical formula weight of 88.06 g. {this is half the given molar mass of 176 g/mol} The molecular formula is C6H8O6. {When the hydrocarbon being burned also contains oxygen, the amount of O can only be determined i ...
... Multiplying al l three numbers by 3 give the empirical formula C3H4O3, which has an empirical formula weight of 88.06 g. {this is half the given molar mass of 176 g/mol} The molecular formula is C6H8O6. {When the hydrocarbon being burned also contains oxygen, the amount of O can only be determined i ...
experiment 1 - University of Delhi
... (chemical or physical change) is carried out by measuring the change in temperature in the calorimeter. Calculations are based upon the first law of thermodynamics according to which the total energy of a system and its surroundings in conserved. In an adiabatic calorimeter the heat exchange with th ...
... (chemical or physical change) is carried out by measuring the change in temperature in the calorimeter. Calculations are based upon the first law of thermodynamics according to which the total energy of a system and its surroundings in conserved. In an adiabatic calorimeter the heat exchange with th ...
Analytical model for melting in a semi-infinite PCM
... as encapsulated PCM particles, the heat transfer surface is large compared to the mass of the PCM. Thus, no heat transfer problem exists inside the particle. The utilisation of LHTS is limited with large heat flux densities. Therefore, the internal enhancement of the heat transfer of PCM is essentia ...
... as encapsulated PCM particles, the heat transfer surface is large compared to the mass of the PCM. Thus, no heat transfer problem exists inside the particle. The utilisation of LHTS is limited with large heat flux densities. Therefore, the internal enhancement of the heat transfer of PCM is essentia ...
Physical Chemistry I – review guide
... their initial states • P-V Work is the work done in a volume change, and it can be expressed as ...
... their initial states • P-V Work is the work done in a volume change, and it can be expressed as ...
Influence of an insulating megaregolith on heat flow and crustal
... figure shows that the surface heat flow increases, whereas crustal temperatures above ...
... figure shows that the surface heat flow increases, whereas crustal temperatures above ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.