Chapter 1: FUNDAMENTAL CONCEPTS OF THERMODYNAMICS
... When a path is completely specified then the change of state is called a process. A system is in thermodynamic equilibrium if there is no change in any thermodynamic properties of a system where it is isolated from its surroundings A quasi static process can be viewed as a sufficiently slow process ...
... When a path is completely specified then the change of state is called a process. A system is in thermodynamic equilibrium if there is no change in any thermodynamic properties of a system where it is isolated from its surroundings A quasi static process can be viewed as a sufficiently slow process ...
MECHANICAL EQUIVALENT OF HEAT In this
... purely historical reasons, as the Mechanical Equivalent of Heat. These days we consider calories and joules simply as different units for energy. The apparatus consists of an aluminum cylinder that can be rotated by a crank, with a thermistor inside the cylinder to measure its temperature. A ...
... purely historical reasons, as the Mechanical Equivalent of Heat. These days we consider calories and joules simply as different units for energy. The apparatus consists of an aluminum cylinder that can be rotated by a crank, with a thermistor inside the cylinder to measure its temperature. A ...
Chapter 8 - Extras Springer
... 8.1.5 Characteristics of Turbulence Turbulence is comprised of irregular, chaotic, three-dimensional fluid motion, but containing coherent structures. Turbulence occurs at high Reynolds numbers, where instabilities give way to chaotic motion. Turbulence is comprised of many scales of eddies, ...
... 8.1.5 Characteristics of Turbulence Turbulence is comprised of irregular, chaotic, three-dimensional fluid motion, but containing coherent structures. Turbulence occurs at high Reynolds numbers, where instabilities give way to chaotic motion. Turbulence is comprised of many scales of eddies, ...
Lecture 3: FIRST LAW OF THERMODYNAMICS
... Calculate the final temperature when cold ice is dropped into hot water. Proceed by assuming a series of processes in which the energy goes into some unspecified storage reservoir The water cools to 0◦ . The ice warms to 0◦ . The ice melts. Finally, the sum of these energies is put back into the sys ...
... Calculate the final temperature when cold ice is dropped into hot water. Proceed by assuming a series of processes in which the energy goes into some unspecified storage reservoir The water cools to 0◦ . The ice warms to 0◦ . The ice melts. Finally, the sum of these energies is put back into the sys ...
3.9-10 Redox titrations
... wire was dissolved in excess of dilute sulphuric acid and the solution made up to 250 cm3 in a standard graduated flask. 25.0 cm3 of this solution was pipetted into a conical flask and needed 25.45 cm3 of 0.02 mol dm-3 KMnO4 for complete oxidation. ...
... wire was dissolved in excess of dilute sulphuric acid and the solution made up to 250 cm3 in a standard graduated flask. 25.0 cm3 of this solution was pipetted into a conical flask and needed 25.45 cm3 of 0.02 mol dm-3 KMnO4 for complete oxidation. ...
Document
... Strategy Water constitutes the surroundings; the pellet is the system. Use qsurr = msΔT to determine the heat absorbed by the water; then use q = CΔT to determine the heat capacity of the metal pellet. mwater = 125 g, swater = 4.184 J/g∙°C, and ΔTwater = 31.3°C – 25.1°C = 6.2°C. The heat absorbed by ...
... Strategy Water constitutes the surroundings; the pellet is the system. Use qsurr = msΔT to determine the heat absorbed by the water; then use q = CΔT to determine the heat capacity of the metal pellet. mwater = 125 g, swater = 4.184 J/g∙°C, and ΔTwater = 31.3°C – 25.1°C = 6.2°C. The heat absorbed by ...
Physics 2
... 49. A system performs work when an amount of heat is added to the system, the corresponding change in the internal energy is . A unique function of the initial and final states (irrespective of the mode of change) is . [CPMT 1981; J & ] ...
... 49. A system performs work when an amount of heat is added to the system, the corresponding change in the internal energy is . A unique function of the initial and final states (irrespective of the mode of change) is . [CPMT 1981; J & ] ...
Thermodynamics Summary
... But how do we express the state of a system? We do this by using properties. The state postulate says that the state of a simple compressible system is specified by two independent intensive properties. This postulate requires some clarification. A system is compressible if the density isn’t constan ...
... But how do we express the state of a system? We do this by using properties. The state postulate says that the state of a simple compressible system is specified by two independent intensive properties. This postulate requires some clarification. A system is compressible if the density isn’t constan ...
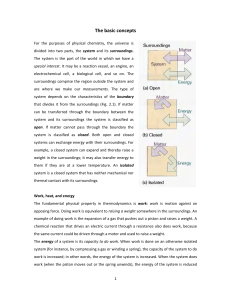
The basic concepts For the purposes of physical chemistry, the
... electron is accelerated from rest through a potential difference of 1 V; the relation between electronvolts and joules is 1 eV = 1.6x10-19 J. Many processes in chemistry have an energy of several electronvolts. Thus, the energy to remove an electron from a sodium atom is close to 5 eV. Calories (cal ...
... electron is accelerated from rest through a potential difference of 1 V; the relation between electronvolts and joules is 1 eV = 1.6x10-19 J. Many processes in chemistry have an energy of several electronvolts. Thus, the energy to remove an electron from a sodium atom is close to 5 eV. Calories (cal ...
Chapter-18
... 18.35 In the emission of thermal T (in kelvins). radiation by an object, apply the relationship between the energy-transfer rate Prad and 18.37 Calculate the net energy the object’s surface area A, transfer rate Pnet of an object emissivity , and surface emitting radiation to its temperature T (in ...
... 18.35 In the emission of thermal T (in kelvins). radiation by an object, apply the relationship between the energy-transfer rate Prad and 18.37 Calculate the net energy the object’s surface area A, transfer rate Pnet of an object emissivity , and surface emitting radiation to its temperature T (in ...
about a variety of material equilibrium conditions
... uko, transported by mole of k-th substance through borders of system. It is easy to be convinced of it, reasoning " from opposite ". Really, for pure of k-th substance as a closed system is fairly the expression duko = δqk – δwk = Tdsko – pdvko. Multiplying all members of this equality on Nk and tak ...
... uko, transported by mole of k-th substance through borders of system. It is easy to be convinced of it, reasoning " from opposite ". Really, for pure of k-th substance as a closed system is fairly the expression duko = δqk – δwk = Tdsko – pdvko. Multiplying all members of this equality on Nk and tak ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.