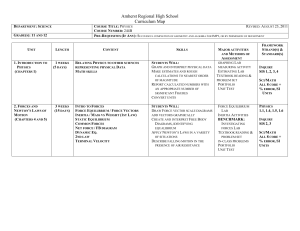
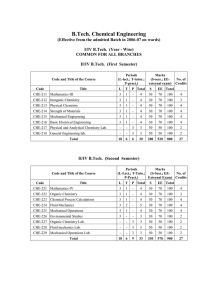
Chapter 12: Engineering Thermodynamics
... There are many effects whose presence during a process renders it irreversible. These include, but are not limited to, the following: heat transfer through a finite temperature difference; unrestrained expansion of a gas or liquid to a lower pressure; spontaneous chemical reaction; mixing of matter ...
... There are many effects whose presence during a process renders it irreversible. These include, but are not limited to, the following: heat transfer through a finite temperature difference; unrestrained expansion of a gas or liquid to a lower pressure; spontaneous chemical reaction; mixing of matter ...
Introduction to Modern Physics PHYX 2710
... Heat (random motion) is a special form of energy that cannot be fully (with complete efficiency) transformed to other forms of energy. This leads to various forms of the Second Law of Thermodynamics. ...
... Heat (random motion) is a special form of energy that cannot be fully (with complete efficiency) transformed to other forms of energy. This leads to various forms of the Second Law of Thermodynamics. ...
numerical modeling and analytical validation for the movement of
... with relatively high volumetric heat capacity makes ATES system more efficient than any other system (e.g. closed system like borehole thermal energy system or BTES) that can be used for this purpose (Kim et al. [2]). The effectiveness of storing the thermal energy in an aquifer depends on the effic ...
... with relatively high volumetric heat capacity makes ATES system more efficient than any other system (e.g. closed system like borehole thermal energy system or BTES) that can be used for this purpose (Kim et al. [2]). The effectiveness of storing the thermal energy in an aquifer depends on the effic ...
Heat Loss Calculations And Principles
... the greater the difference in temperatures, the greater will be the heat flow. There are three types of heat transfer: 1. Via Conduction - This occurs when two objects are in direct contact, for example the air against a window or the soil against a foundation. In buildings, this is typically the mo ...
... the greater the difference in temperatures, the greater will be the heat flow. There are three types of heat transfer: 1. Via Conduction - This occurs when two objects are in direct contact, for example the air against a window or the soil against a foundation. In buildings, this is typically the mo ...
Sample pages 2 PDF
... More complex to describe is the non-homogeneous system, in which some of the variables, for example, the temperature or the pressure, vary from point to point. To study them, one must divide the system into parts that are small enough to be able to be considered homogeneous. We shall not deal with a ...
... More complex to describe is the non-homogeneous system, in which some of the variables, for example, the temperature or the pressure, vary from point to point. To study them, one must divide the system into parts that are small enough to be able to be considered homogeneous. We shall not deal with a ...
The Helmholtz Function
... The quantity G is tabulated for a variety of chemical reactions and other processes. G = H - TS In most work we do not need to choose a reference point as, usually, only changes in the quantities are of interest. {One source of tabulations of thermodynamic properties is the CRC Handbook of Chem ...
... The quantity G is tabulated for a variety of chemical reactions and other processes. G = H - TS In most work we do not need to choose a reference point as, usually, only changes in the quantities are of interest. {One source of tabulations of thermodynamic properties is the CRC Handbook of Chem ...
Meaning of Entropy in Classical Thermodynamics
... he arrived at the cyclic integral, Q τ , that equals zero for a reversible cycle. One of the critics of the foundations of Thermodynamics, Truesdell, attacks the rather circular definition of irreversible processes offered by Clausius: irreversible processes are those processes that are not reversib ...
... he arrived at the cyclic integral, Q τ , that equals zero for a reversible cycle. One of the critics of the foundations of Thermodynamics, Truesdell, attacks the rather circular definition of irreversible processes offered by Clausius: irreversible processes are those processes that are not reversib ...
Fundamental Concepts, Definitions and Zeroth
... It is the pressure of a fluid contained in a closed vessel. It is always more than atmospheric pressure. It is measured by an instrument called pressure gauge (such as Bourden’s pressure gauge). The gauge measures pressure of the fluid (liquid and gas) flowing through a pipe or duct, boiler etc. irr ...
... It is the pressure of a fluid contained in a closed vessel. It is always more than atmospheric pressure. It is measured by an instrument called pressure gauge (such as Bourden’s pressure gauge). The gauge measures pressure of the fluid (liquid and gas) flowing through a pipe or duct, boiler etc. irr ...
Temporal and spatial dispersion of human body temperature during
... All patients suffered from severe cardiac diseases or abnormalities that provided an indication for surgical reconstruction (American Society of Anesthesiologists Classification, ASA IV) and received open-heart surgery. Exclusion criteria were met if a proper fixation of the cranial double sensor th ...
... All patients suffered from severe cardiac diseases or abnormalities that provided an indication for surgical reconstruction (American Society of Anesthesiologists Classification, ASA IV) and received open-heart surgery. Exclusion criteria were met if a proper fixation of the cranial double sensor th ...
Fluids and Thermo Review
... fluids at rest, such as the pressure of a fluid at a particular depth, or the buoyant force acting on an object in a fluid. Archimedes principle states that the buoyant force acting on an object in a fluid is equal to the weight of the fluid displaced by the object Hydrodynamics is the study of flui ...
... fluids at rest, such as the pressure of a fluid at a particular depth, or the buoyant force acting on an object in a fluid. Archimedes principle states that the buoyant force acting on an object in a fluid is equal to the weight of the fluid displaced by the object Hydrodynamics is the study of flui ...
托福TPO15阅读word版下载一
... maintain a body temperature of between 25 and 26°C (77-79°F) in seawater that is only 8°C (46.4°F). Accomplishing this feat requires adaptations both to generate heat in the turtle’s body and to keep it from escaping into the surrounding waters. Leatherbacks apparently do not generate internal heat ...
... maintain a body temperature of between 25 and 26°C (77-79°F) in seawater that is only 8°C (46.4°F). Accomplishing this feat requires adaptations both to generate heat in the turtle’s body and to keep it from escaping into the surrounding waters. Leatherbacks apparently do not generate internal heat ...
unit 61: engineering thermodynamics
... Heat transfer occurs because one place is hotter than another. Under normal circumstances, heat will only flow from a hot body to a cold body by virtue of the temperature difference. There are 3 mechanisms for this, Conduction, convection and radiation. You do not need to study the laws governing co ...
... Heat transfer occurs because one place is hotter than another. Under normal circumstances, heat will only flow from a hot body to a cold body by virtue of the temperature difference. There are 3 mechanisms for this, Conduction, convection and radiation. You do not need to study the laws governing co ...
Thermodynamics of dilute gases
... independent of their physical properties and the special kind of contact. Just this property is used to bring a substance to a given temperature, namely, by surrounding it with a heat bath. Then, by definition, substance and heat bath have the same temperature. To measure the temperature one can emp ...
... independent of their physical properties and the special kind of contact. Just this property is used to bring a substance to a given temperature, namely, by surrounding it with a heat bath. Then, by definition, substance and heat bath have the same temperature. To measure the temperature one can emp ...
Document
... Adiabatic processes for an ideal gas • In an adiabatic process, no heat is transferred in or out of the gas, so Q = 0. • Shown is a pV-diagram for an adiabatic expansion. • As the gas expands, it does positive work W on its environment, so its internal energy decreases, and its temperature drops. • ...
... Adiabatic processes for an ideal gas • In an adiabatic process, no heat is transferred in or out of the gas, so Q = 0. • Shown is a pV-diagram for an adiabatic expansion. • As the gas expands, it does positive work W on its environment, so its internal energy decreases, and its temperature drops. • ...