Electronic Homework Problems Questions and Problems Key Words
... is titrated by 1 mole of a strong base (such as KOH) at 25°C. Calculate the heats of combustion for the following reactions from the standard enthalpies of formation listed in Appendix 3: (a) 2H 2 (g) 1 O 2 (g) ¡ 2H 2O(l) (b) 2C 2H 2 (g) 1 5O 2 (g) ¡ 4CO 2 (g) 1 2H 2O(l) Calculate the heats of combu ...
... is titrated by 1 mole of a strong base (such as KOH) at 25°C. Calculate the heats of combustion for the following reactions from the standard enthalpies of formation listed in Appendix 3: (a) 2H 2 (g) 1 O 2 (g) ¡ 2H 2O(l) (b) 2C 2H 2 (g) 1 5O 2 (g) ¡ 4CO 2 (g) 1 2H 2O(l) Calculate the heats of combu ...
Engines and the Second Law of Thermodynamics
... energy is conserved. However, the absence of the process illustrated above indicates that conservation of energy is not the whole story. If it were, movies run backwards would look perfectly normal to us! Copyright © 2009 Pearson Education, Inc. ...
... energy is conserved. However, the absence of the process illustrated above indicates that conservation of energy is not the whole story. If it were, movies run backwards would look perfectly normal to us! Copyright © 2009 Pearson Education, Inc. ...
Section 8: Electronic Transport
... propagation in periodic structures that, when a wave passes through a periodic lattice, it continues propagating indefinitely without scattering. The effect of the atoms in the lattice is to absorb energy from the wave and radiate it back, so that the net result is that the wave continues without mo ...
... propagation in periodic structures that, when a wave passes through a periodic lattice, it continues propagating indefinitely without scattering. The effect of the atoms in the lattice is to absorb energy from the wave and radiate it back, so that the net result is that the wave continues without mo ...
ISM_CH26 - Academic Program Pages
... 1. (a) The charge that passes through any cross section is the product of the current and time. Since 4.0 min = (4.0 min)(60 s/min) = 240 s, q = it = (5.0 A)(240 s) = 1.2 103 C. (b) The number of electrons N is given by q = Ne, where e is the magnitude of the charge on an electron. Thus, N = q/e = ...
... 1. (a) The charge that passes through any cross section is the product of the current and time. Since 4.0 min = (4.0 min)(60 s/min) = 240 s, q = it = (5.0 A)(240 s) = 1.2 103 C. (b) The number of electrons N is given by q = Ne, where e is the magnitude of the charge on an electron. Thus, N = q/e = ...
transparencies
... The low frequency value of M' is zero and represents a lack of the restoring force for the electric field induced mobile Tl ions. As frequency increases, each ion moves a shorter and shorter distance until finally the electric field changes so rapidly that the ions only oscillate within the confinem ...
... The low frequency value of M' is zero and represents a lack of the restoring force for the electric field induced mobile Tl ions. As frequency increases, each ion moves a shorter and shorter distance until finally the electric field changes so rapidly that the ions only oscillate within the confinem ...
SUPAPAC Plate Heat Exchangers
... Rycroft Plate Heat Exchangers are pressure tested at the factory according to the specification. As a result the heat exchanger is ready to use when it arrives on site. The nameplate shows the tightening distance between the fixed and loose end plate. The tightening dimension is the minimum distance ...
... Rycroft Plate Heat Exchangers are pressure tested at the factory according to the specification. As a result the heat exchanger is ready to use when it arrives on site. The nameplate shows the tightening distance between the fixed and loose end plate. The tightening dimension is the minimum distance ...
Synthesis of a High Temperature Superconductor
... This property was first discovered by Heike Kamerlingh Onnes in 1911 when he observed the resistance of a column of Mercury dropped to zero when cooled to about 4K. In the spring of 1911, Onnes began his studies of electrical conductivity of metals at low temperature. Physicists at the time knew tha ...
... This property was first discovered by Heike Kamerlingh Onnes in 1911 when he observed the resistance of a column of Mercury dropped to zero when cooled to about 4K. In the spring of 1911, Onnes began his studies of electrical conductivity of metals at low temperature. Physicists at the time knew tha ...
Thermodynamics: the Second Law
... engines have both a hot source and a cold sink; some heat is always discarded into the cold sink and is not converted into work. Another everyday observation: a ball at rest on a surface has never been observed to leap spontaneously upwards. An upward leap of the ball would be equivalent to the conv ...
... engines have both a hot source and a cold sink; some heat is always discarded into the cold sink and is not converted into work. Another everyday observation: a ball at rest on a surface has never been observed to leap spontaneously upwards. An upward leap of the ball would be equivalent to the conv ...
Global distribution of the lithosphere-asthenosphere
... of lithospheric thickness are based on the newly proposed finite half-space (FHS) model. Unlike the half-space cooling (HSC) and the plate models the FHS model takes into account effects of buffered solidification at the lower boundary of the lithosphere and assumes that the vertical domain for down ...
... of lithospheric thickness are based on the newly proposed finite half-space (FHS) model. Unlike the half-space cooling (HSC) and the plate models the FHS model takes into account effects of buffered solidification at the lower boundary of the lithosphere and assumes that the vertical domain for down ...
Lecture 6 - web page for staff
... • The distance between them (d) is sufficient small, so that the electrons in the left-side semiconductor may transport across the barrier and move to the right-side semiconductor even the electron energy is much less than the barrier height. • This process is called “quantum tunneling process”. ...
... • The distance between them (d) is sufficient small, so that the electrons in the left-side semiconductor may transport across the barrier and move to the right-side semiconductor even the electron energy is much less than the barrier height. • This process is called “quantum tunneling process”. ...
ENTROPY
... pressure, it is offered zero resistance. Hence, no work is one by or upon the gas. If this sounds unconvincing, consider ∫PextdV. Since the external pressure is constant, we take it out of the integral, and since the external pressure is zero, the whole term is zero by itself. w, then, too becomes z ...
... pressure, it is offered zero resistance. Hence, no work is one by or upon the gas. If this sounds unconvincing, consider ∫PextdV. Since the external pressure is constant, we take it out of the integral, and since the external pressure is zero, the whole term is zero by itself. w, then, too becomes z ...
Thermal Simulation for a Room with Solid and One
... In this analysis, the three processes of heat transfer (conduction, convection and radiation) were combined. As shown in Fig. 3, pure conduction occurs through the solid part of the bricks while the heat transfer by convection and radiation occurs between the ambient air and outer and inner wall sur ...
... In this analysis, the three processes of heat transfer (conduction, convection and radiation) were combined. As shown in Fig. 3, pure conduction occurs through the solid part of the bricks while the heat transfer by convection and radiation occurs between the ambient air and outer and inner wall sur ...
effect of pool boili g usi g al2o3-water ad fe2o3-water a
... The nucleate boiling regime can be considered one of the most effective ways to obtain a great amount of heat exchange in a relatively small area. The limit of nucleate boiling is characterized by the critical heat flux (CHF), and occurs when the liquid film under the bubbles evaporates completely, ...
... The nucleate boiling regime can be considered one of the most effective ways to obtain a great amount of heat exchange in a relatively small area. The limit of nucleate boiling is characterized by the critical heat flux (CHF), and occurs when the liquid film under the bubbles evaporates completely, ...
Kelvin scale
... The kelvin is formally defined as a unit of thermodynamic (i.e., absolute) temperature equal to 1/273.16 of the thermodynamic temperature of the triple point of water—the temperature at which steam, ice and water are at equilibrium. This unit is named for the man who first proposed using this scale, ...
... The kelvin is formally defined as a unit of thermodynamic (i.e., absolute) temperature equal to 1/273.16 of the thermodynamic temperature of the triple point of water—the temperature at which steam, ice and water are at equilibrium. This unit is named for the man who first proposed using this scale, ...
Heat Transfer Enhancement in a Circular Tube Twisted with Swirl
... has been widely applied to heat exchanger applications in refrigeration, automobile, process industries etc. The goal of enhanced heat transfer is to encourage or accommodate high heat fluxes. This result in reduction of heat exchanger size, which generally leads to less capital cost. Another advant ...
... has been widely applied to heat exchanger applications in refrigeration, automobile, process industries etc. The goal of enhanced heat transfer is to encourage or accommodate high heat fluxes. This result in reduction of heat exchanger size, which generally leads to less capital cost. Another advant ...
Investigation on Numerical Modeling of Water Vapour Condensation
... tion of water vapour from a flue gas with high CO2 concentration could provide such useful technical data for designers. Naturally, condensation happens whenever the vapour temperature is decreased by cooling until it reaches the saturation temperature Tsat at the operational pressure and usually th ...
... tion of water vapour from a flue gas with high CO2 concentration could provide such useful technical data for designers. Naturally, condensation happens whenever the vapour temperature is decreased by cooling until it reaches the saturation temperature Tsat at the operational pressure and usually th ...
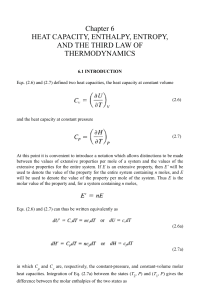
Chapter 6 HEAT CAPACITY, ENTHALPY, ENTROPY, AND
... The variation of Cv with T/ E is shown in Fig. 6.2, which shows that as T/ E (and hence T) increases, Cv → 3R in agreement with Dulong and Petit’s law, and as T → 0, Cv → 0, which is in agreement with experimental observation. The actual value of E for any element and its vibration frequency, v, ar ...
... The variation of Cv with T/ E is shown in Fig. 6.2, which shows that as T/ E (and hence T) increases, Cv → 3R in agreement with Dulong and Petit’s law, and as T → 0, Cv → 0, which is in agreement with experimental observation. The actual value of E for any element and its vibration frequency, v, ar ...
NOTAT
... Many gas properties can be related to a concept of gas as molecules flying around in a room. The speed, and thus energy, increases with temperature. The distance between the molecules is very large, so that there is no interaction between them except for random and elastic collisions. The pressure i ...
... Many gas properties can be related to a concept of gas as molecules flying around in a room. The speed, and thus energy, increases with temperature. The distance between the molecules is very large, so that there is no interaction between them except for random and elastic collisions. The pressure i ...
Chapter 5 Outline 1213 full
... than in (a). The value of ΔE is the same for both processes even though the values of q and w in (a) are different from the values of q and w in (b). ...
... than in (a). The value of ΔE is the same for both processes even though the values of q and w in (a) are different from the values of q and w in (b). ...