Heat of Reaction
... combusted to heat 50 gallon (189.3 L) of water from 50F (10C) to 140F (60C) ? Specific heat H2O = ...
... combusted to heat 50 gallon (189.3 L) of water from 50F (10C) to 140F (60C) ? Specific heat H2O = ...
MgO thermo lab
... It is difficult to directly measure the heat absorbed or evolved in some reactions because of the difficulty in performing the reaction and obtaining accurate data or the reaction occurs too slowly to produce a noticeable temperature change. The combustion on magnesium occurs so rapidly that it is d ...
... It is difficult to directly measure the heat absorbed or evolved in some reactions because of the difficulty in performing the reaction and obtaining accurate data or the reaction occurs too slowly to produce a noticeable temperature change. The combustion on magnesium occurs so rapidly that it is d ...
APPLICATIONS OF MICROCALORIMETRY IN STABILITY STUDIES INTRODUCTION:
... A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Bomb calorimeters have to withstand the large pressure within the calorimeter as the reaction is being measured. Electrical energy is used to ignite the fuel; as the fuel is ...
... A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Bomb calorimeters have to withstand the large pressure within the calorimeter as the reaction is being measured. Electrical energy is used to ignite the fuel; as the fuel is ...
Chapter 22
... 1. A heat engine performs 1500 J of work each cycle and is 20 % efficient. (a) Determine how much heat is taken in during each cycle and (b) how heat is exhausted each cycle. Ans. (a) 7500 J (b) 6000 J 2. A particular heat engine absorbs 1.5 MJ from the hot reservoir 327 οC and exhausts 1 MJ to the ...
... 1. A heat engine performs 1500 J of work each cycle and is 20 % efficient. (a) Determine how much heat is taken in during each cycle and (b) how heat is exhausted each cycle. Ans. (a) 7500 J (b) 6000 J 2. A particular heat engine absorbs 1.5 MJ from the hot reservoir 327 οC and exhausts 1 MJ to the ...
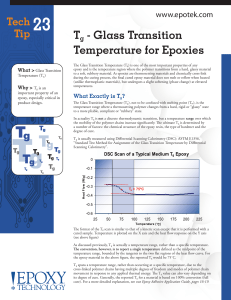
Tg - Glass Transition Temperature for Epoxies
... The basic relationship Modulus has to adhesives is: the higher the Tg , the higher the cross-linked density and the higher the modulus. As an epoxy rises above its Tg , the storage modulus drops. This is indicative of the change from a rigid to compliant state. A high Tg along with a high storage mo ...
... The basic relationship Modulus has to adhesives is: the higher the Tg , the higher the cross-linked density and the higher the modulus. As an epoxy rises above its Tg , the storage modulus drops. This is indicative of the change from a rigid to compliant state. A high Tg along with a high storage mo ...
notes - superTALLteacher
... The smaller the specific heat the less energy it takes the substance to feel hot The larger the specific heat the more energy it takes to heat a substance up The smaller the specific heat the less time it takes the substance to cool off The larger the specific heat the longer time it t ...
... The smaller the specific heat the less energy it takes the substance to feel hot The larger the specific heat the more energy it takes to heat a substance up The smaller the specific heat the less time it takes the substance to cool off The larger the specific heat the longer time it t ...
3-10-09 Thermodynamics
... somebody bumps the table they rest on? – They fall and most likely will not all land with the same side up ...
... somebody bumps the table they rest on? – They fall and most likely will not all land with the same side up ...
Physics Perspectives of Environments
... When vapor becomes water by cooling, it looks like the entropy decreases. This is because it is an open system. In terms of the larger system, the entropy increases by the heat radiation. ...
... When vapor becomes water by cooling, it looks like the entropy decreases. This is because it is an open system. In terms of the larger system, the entropy increases by the heat radiation. ...
File
... Comparing Ectotherms and Endotherms Large ectotherms run into trouble, however, if temperatures get very cold at night or stay cold for long periods. A large animal takes a long time to warm up in the sun after a cold night. 15. Endotherms survive more easily during cool nights or in cold weather be ...
... Comparing Ectotherms and Endotherms Large ectotherms run into trouble, however, if temperatures get very cold at night or stay cold for long periods. A large animal takes a long time to warm up in the sun after a cold night. 15. Endotherms survive more easily during cool nights or in cold weather be ...
Apparatus to measure high-temperature thermal conductivity and
... details of instrument fabrication, the method of calibration, and typical measurements on test samples are described. The apparatus can also be used to measure the Seebeck coefficient in the same temperature range. As an example we report the thermal properties of CrSi2, which is a potential candida ...
... details of instrument fabrication, the method of calibration, and typical measurements on test samples are described. The apparatus can also be used to measure the Seebeck coefficient in the same temperature range. As an example we report the thermal properties of CrSi2, which is a potential candida ...
WBL6_Lecture_Ch10-2djgx21
... the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permitted. The work and materials from it should never be made available to students exc ...
... the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permitted. The work and materials from it should never be made available to students exc ...
Thermodynamics // Homework #3 Closed System Energy Analysis 1
... and 700°F. Nitrogen is now allowed to cool at constant pressure until the temperature drops to 200°F. Using specific heats at the average temperature, determine the amount of heat loss. {284.2 Btu} 14. A piston–cylinder device contains 0.8 kg of nitrogen initially at 100 kPa and 27°C. The nitrogen i ...
... and 700°F. Nitrogen is now allowed to cool at constant pressure until the temperature drops to 200°F. Using specific heats at the average temperature, determine the amount of heat loss. {284.2 Btu} 14. A piston–cylinder device contains 0.8 kg of nitrogen initially at 100 kPa and 27°C. The nitrogen i ...
Chapter 13 Heat and Temperature
... Relating Temperature to Energy Transfer The feeling associated with temperature difference results from energy transfer If you hold an ice cube the energy of the ice cube is less then the energy of your hands. When the molecules of your hand come into contact with the ice, they cause the ice molecu ...
... Relating Temperature to Energy Transfer The feeling associated with temperature difference results from energy transfer If you hold an ice cube the energy of the ice cube is less then the energy of your hands. When the molecules of your hand come into contact with the ice, they cause the ice molecu ...
vital signs - Georgetown ISD
... or higher Causes: stress, anxiety, obesity, high Na intake, aging, kidney disease, thyroid deficiency, vascular conditions (arteriosclerosis) HTN not treated will lead to kidney failure, stroke, heart disease ...
... or higher Causes: stress, anxiety, obesity, high Na intake, aging, kidney disease, thyroid deficiency, vascular conditions (arteriosclerosis) HTN not treated will lead to kidney failure, stroke, heart disease ...
MARS: OCCURRENCE OF LIQUID WATER The purpose of this
... out during the Martian night, the morning frost layer would be only 10-20 fxm thick. From Table I with A = 676 cal/gm, the lifetime of such a frost at —10 °C would be several minutes, and since the frost is likely to spend more time than this in warming from —10 °C to 0 °C, it will probably disappea ...
... out during the Martian night, the morning frost layer would be only 10-20 fxm thick. From Table I with A = 676 cal/gm, the lifetime of such a frost at —10 °C would be several minutes, and since the frost is likely to spend more time than this in warming from —10 °C to 0 °C, it will probably disappea ...
HEAT OF FUSION AND MECHANICAL EQUIVALENT OF HEAT
... work against the frictional force between two surfaces that are rubbing together; these surfaces will become hot, and the temperature difference between the rubbing surfaces and the rest of the system will cause heat to flow within the system. By measuring the temperature increase of the system as a ...
... work against the frictional force between two surfaces that are rubbing together; these surfaces will become hot, and the temperature difference between the rubbing surfaces and the rest of the system will cause heat to flow within the system. By measuring the temperature increase of the system as a ...
Outside-class project#9a questions
... (1) List the stages of development of an ordinary cell (air mass) thunderstorm and the typical period of time associated with the stages of development for a single thunderstorm. Explain why ordinary cell thunderstorms tend to dissipate much sooner than multicell storms. ...
... (1) List the stages of development of an ordinary cell (air mass) thunderstorm and the typical period of time associated with the stages of development for a single thunderstorm. Explain why ordinary cell thunderstorms tend to dissipate much sooner than multicell storms. ...
Thermally Conductive Aluminum Tape
... All properties are typical values and should not be used for writing specifications. ...
... All properties are typical values and should not be used for writing specifications. ...
The Skin - Education Service Center, Region 2
... integumentary system; respiratory system to get oxygen and remove carbon dioxide. circulatory system to deliver ...
... integumentary system; respiratory system to get oxygen and remove carbon dioxide. circulatory system to deliver ...
Document
... An amount of heat equal to 2500 J is added to a system, and 1800 J of work is done on the system. What is the change in internal energy of the system? A. ...
... An amount of heat equal to 2500 J is added to a system, and 1800 J of work is done on the system. What is the change in internal energy of the system? A. ...
Customer Application Brief General Industrial Filtration in Process
... Many industrial processes typically use water to remove heat generated from the process. Common applications that require process cooling water include electronics manufacturing, power plant condensate systems, and thin film manufacturing; however, virtually every manufacturing plant employs a cooli ...
... Many industrial processes typically use water to remove heat generated from the process. Common applications that require process cooling water include electronics manufacturing, power plant condensate systems, and thin film manufacturing; however, virtually every manufacturing plant employs a cooli ...
module 7
... applications. In parallel flow both the hot and cold streams enter the heat exchanger at the same end and travel to the opposite end in parallel streams. Energy is transferred along the length from the hot to the cold fluid so the outlet temperatures asymptotically approach each other. In a counter ...
... applications. In parallel flow both the hot and cold streams enter the heat exchanger at the same end and travel to the opposite end in parallel streams. Energy is transferred along the length from the hot to the cold fluid so the outlet temperatures asymptotically approach each other. In a counter ...
Paper
... investigation of the problem. Some of them use the numerical modeling of heat transfer during melt spinning process. The work [2] is the good example of such approach. The heat transfer both inside the melt and in the substrate is incorporated directly in the numerical solutions. The calculations ha ...
... investigation of the problem. Some of them use the numerical modeling of heat transfer during melt spinning process. The work [2] is the good example of such approach. The heat transfer both inside the melt and in the substrate is incorporated directly in the numerical solutions. The calculations ha ...
Chapter 5 PPT 2 - Kawameeh Middle School
... Expansion, or increase in size of a substance caused by ...
... Expansion, or increase in size of a substance caused by ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.