21.3 Administering Heat/Cold Applications
... Compresses, Packs, and Soaks Dry Cold: Used Mainly to reduce body temperature and examples include: Ice Bags, Ice Collars, and Hypothermia Blankets ...
... Compresses, Packs, and Soaks Dry Cold: Used Mainly to reduce body temperature and examples include: Ice Bags, Ice Collars, and Hypothermia Blankets ...
temp, water balance and the urinary sytem
... -Create a tubular fluid by filtering the blood under pressure through the glomerulus -Filtrate contains many small molecules, in addition to water and waste products -Most of these molecules and water are reabsorbed into the blood -Waste products are eliminated from the body in the form of urine ...
... -Create a tubular fluid by filtering the blood under pressure through the glomerulus -Filtrate contains many small molecules, in addition to water and waste products -Most of these molecules and water are reabsorbed into the blood -Waste products are eliminated from the body in the form of urine ...
callister7e_sm_ch10_..
... (a) In order to convert from (martensite + ferrite + bainite) to (martensite + ferrite + pearlite + bainite) it is necessary to heat above about 720°C, allow complete austenitization, then cool to room temperature at a rate between 0.02 and 0.006°C/s. (b) To convert from (martensite + ferrite + bain ...
... (a) In order to convert from (martensite + ferrite + bainite) to (martensite + ferrite + pearlite + bainite) it is necessary to heat above about 720°C, allow complete austenitization, then cool to room temperature at a rate between 0.02 and 0.006°C/s. (b) To convert from (martensite + ferrite + bain ...
Specific Heat and Calculating Heat Absorbed - Varga
... The temperature that you want to raise a substance to is equally important as the mass of the substance that you have. Temperature change is often given the symbol Δt, and is equal to final temperature – initial temperature. ...
... The temperature that you want to raise a substance to is equally important as the mass of the substance that you have. Temperature change is often given the symbol Δt, and is equal to final temperature – initial temperature. ...
Waste Heat Recovery from PV Panels FINAL PRESENTATION
... clear days was on average ~104F (when pump was running) and 122F (when pump was off). ...
... clear days was on average ~104F (when pump was running) and 122F (when pump was off). ...
Consider a rigid tank with a movable piston
... Air-Standard Assumptions: 1) The working fluid is air, which continuously circulates in a closed loop and always behaves as an ideal gas. 2) All the processes that make up the cycle are internally reversible. 3) The combustion process is replaced by a heat-addition process from an external source. 4 ...
... Air-Standard Assumptions: 1) The working fluid is air, which continuously circulates in a closed loop and always behaves as an ideal gas. 2) All the processes that make up the cycle are internally reversible. 3) The combustion process is replaced by a heat-addition process from an external source. 4 ...
Chapters 12-15 Thermodynamics
... temperature, pressure, volume • The macroscopic parameters reflect the average behavior of the microscopic constituents of the system, for example, the velocities of the molecules which cannot be directly measured • Newton’s laws cannot be used to solve problems involving 1023 particles ...
... temperature, pressure, volume • The macroscopic parameters reflect the average behavior of the microscopic constituents of the system, for example, the velocities of the molecules which cannot be directly measured • Newton’s laws cannot be used to solve problems involving 1023 particles ...
5a - homeostasis and feedback
... • Less common as it can get out of control and will not stop until stimulus is removed • Increases chaos in body systems • Examples – blood clotting and child birth ...
... • Less common as it can get out of control and will not stop until stimulus is removed • Increases chaos in body systems • Examples – blood clotting and child birth ...
File 2 - College of Science | Oregon State University
... state going through exactly the same intermediate states as when the gas was expanded – or going back along the same path In the p-V space. ...
... state going through exactly the same intermediate states as when the gas was expanded – or going back along the same path In the p-V space. ...
thermodynamics
... food off, how much equivalent mechanical work would you have to do (in Joules please)? (Note: 4.186 J = 1 cal. The definition of a “cal” is the amount of heat required to raise 1 g of water 1 degree celsius–i.e. the specific heat. Memorize this number.) 1500 Cal A (1000 cal/1 Cal) A (4.186 J/1cal) = ...
... food off, how much equivalent mechanical work would you have to do (in Joules please)? (Note: 4.186 J = 1 cal. The definition of a “cal” is the amount of heat required to raise 1 g of water 1 degree celsius–i.e. the specific heat. Memorize this number.) 1500 Cal A (1000 cal/1 Cal) A (4.186 J/1cal) = ...
Unit 6: Human Health And Physiology
... 6.5.8 State that homeostasis involves maintaining the internal environment at a constant level or between narrow limits. • Homeostatic Factors: – 1) blood pH – 2) oxygen and CO2 concentrations – 3) blood glucose – 4) body temperature – 5) water balance ...
... 6.5.8 State that homeostasis involves maintaining the internal environment at a constant level or between narrow limits. • Homeostatic Factors: – 1) blood pH – 2) oxygen and CO2 concentrations – 3) blood glucose – 4) body temperature – 5) water balance ...
23–1 Specialized Tissues in Plants - Mrs. Della
... maintaining homeostasis in many vertebrates, particularly endotherm in habitats where temperature varies widely with time of day and with season. Most fishes, amphibians, and reptiles are ectotherms— organisms whose body temperatures are controlled primarily by picking up heat from, or losing heat t ...
... maintaining homeostasis in many vertebrates, particularly endotherm in habitats where temperature varies widely with time of day and with season. Most fishes, amphibians, and reptiles are ectotherms— organisms whose body temperatures are controlled primarily by picking up heat from, or losing heat t ...
Heat Calculations with Specific Heat
... • EX. How much heat is necessary to totally melt 5 g of ice at 0 C to liquid water at 0 C? • EX. How much heat is necessary to change 5 g of water at 100 C to steam at 100 C? ...
... • EX. How much heat is necessary to totally melt 5 g of ice at 0 C to liquid water at 0 C? • EX. How much heat is necessary to change 5 g of water at 100 C to steam at 100 C? ...
ENT 211 Tutorial Week 1
... Why is Heat Transfer a nonequilibrium phenomenon? Heat transfer is a non-equilibrium phenomena since in a system that is in equilibrium there can be no temperature differences and thus no heat flow. ...
... Why is Heat Transfer a nonequilibrium phenomenon? Heat transfer is a non-equilibrium phenomena since in a system that is in equilibrium there can be no temperature differences and thus no heat flow. ...
FIXED TEMPERATURE HEAT DETECTOR 70°C WATER
... Ease of installation and wiring connection reduces installation costs ...
... Ease of installation and wiring connection reduces installation costs ...
Full PDF
... Folk(1974) demonstrated that the main natural physical environmental factors affecting livestock are air temperature, relative humidity, radiant heat, precipitation, atmospheric pressure, UV-light, wind velocity and dust. Impact of hot climate on animal productivity causes maintenance requirement to ...
... Folk(1974) demonstrated that the main natural physical environmental factors affecting livestock are air temperature, relative humidity, radiant heat, precipitation, atmospheric pressure, UV-light, wind velocity and dust. Impact of hot climate on animal productivity causes maintenance requirement to ...
Here is an example formatted abstract
... Decadal change of the deep and upper ocean heat content of the north-east Atlantic KING, MCDONAGH, GARRY We examine the vertical distribution of trends in heat content of the north-east basin of the Atlantic Ocean since the late 1980s. The 2010 analysis of Purkey and Johnson identified this basin as ...
... Decadal change of the deep and upper ocean heat content of the north-east Atlantic KING, MCDONAGH, GARRY We examine the vertical distribution of trends in heat content of the north-east basin of the Atlantic Ocean since the late 1980s. The 2010 analysis of Purkey and Johnson identified this basin as ...
Specific Heat Capacity - Tasker Milward
... Specific Heat Capacity • The specific heat capacity is the amount of energy required to increase the temperature of 1kg of a substance by 1˚C • We will calculate the specific heat capacity of water by heating it with an electrical heater and measuring the energy required for a fixed temperature ris ...
... Specific Heat Capacity • The specific heat capacity is the amount of energy required to increase the temperature of 1kg of a substance by 1˚C • We will calculate the specific heat capacity of water by heating it with an electrical heater and measuring the energy required for a fixed temperature ris ...
SPECIFIC HEAT CAPACITY OF WATER
... Where the c is the specific heat of water and ΔT is the temperature difference for water before and after heating. If the water and the resistor are isolated then ...
... Where the c is the specific heat of water and ΔT is the temperature difference for water before and after heating. If the water and the resistor are isolated then ...
Specific Heat WS #2 - My Chemistry Class
... 8750 J of heat are applied to a piece of aluminum, causing a 56 °C increase in its temperature. The specific heat of aluminum is 0.9025 J/g °C. What is the mass of the aluminum? ...
... 8750 J of heat are applied to a piece of aluminum, causing a 56 °C increase in its temperature. The specific heat of aluminum is 0.9025 J/g °C. What is the mass of the aluminum? ...
Chapter 40 Presentation
... 5. Adjusting Metabolism There are a variety of ways by which animals can control their body temperature by changing their metabolic activity. In some mammals, hormones can stimulate mitochondria to generate heat instead of ATP--non-shivering thermogenesis. ...
... 5. Adjusting Metabolism There are a variety of ways by which animals can control their body temperature by changing their metabolic activity. In some mammals, hormones can stimulate mitochondria to generate heat instead of ATP--non-shivering thermogenesis. ...
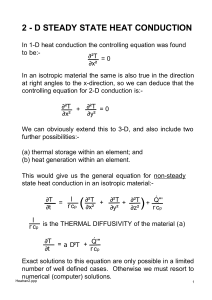
2 - D STEADY STATE HEAT CONDUCTION
... then T1 + T2 + T3 + T4 - 4To = l These equations may be solved by spreadsheet iteration 'updating' each temperature 'cell' in sequence . Alternatively an array of simultaneous equations may be set up and solved by Gaussian elimination, either by hand (!) or by computer. BOUNDARY CONDITIONS The above ...
... then T1 + T2 + T3 + T4 - 4To = l These equations may be solved by spreadsheet iteration 'updating' each temperature 'cell' in sequence . Alternatively an array of simultaneous equations may be set up and solved by Gaussian elimination, either by hand (!) or by computer. BOUNDARY CONDITIONS The above ...
student powerpoint 3
... • In a body at rest, radiation is the primary method for discharging the resting body’s excess heat. • At normal room temperature (21-25°C), the nude body loses about 60% of its excess heat by radiation. • The heat is given off in the form of infrared rays, which are a type of electromagnetic waves. ...
... • In a body at rest, radiation is the primary method for discharging the resting body’s excess heat. • At normal room temperature (21-25°C), the nude body loses about 60% of its excess heat by radiation. • The heat is given off in the form of infrared rays, which are a type of electromagnetic waves. ...
Recitation 3.2 Temperature/Heat
... bonded together. As the temperature changed, the strip would bend due to the different rates of expansion. An example is provided. Heat it with a hair dryer, and then put in front of the air conditioner to see what happens. If the material with the higher coefficient of expansion were on the left, w ...
... bonded together. As the temperature changed, the strip would bend due to the different rates of expansion. An example is provided. Heat it with a hair dryer, and then put in front of the air conditioner to see what happens. If the material with the higher coefficient of expansion were on the left, w ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.