How to Balance Chemical Equations
... Since the hydrogen atoms come in pairs we need ____ pairs to make 4. ...
... Since the hydrogen atoms come in pairs we need ____ pairs to make 4. ...
*6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing
... *6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing Chemical Change *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that al ...
... *6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing Chemical Change *Changes in matter can be described in terms of physical changes and chemical changes. *Chemical reactions involve changes in properties and changes in energy that you can often observe. Physical change-any change that al ...
Process Hazard Analysis and Automation Sandra Y. Smith
... potential hazards associated with chemical processing and handling – A tool to assist in making decisions for improving safety and reducing the consequences of unplanned releases of hazardous chemicals – Directed toward analyzing potential causes and consequences of fires, explosions, releases of to ...
... potential hazards associated with chemical processing and handling – A tool to assist in making decisions for improving safety and reducing the consequences of unplanned releases of hazardous chemicals – Directed toward analyzing potential causes and consequences of fires, explosions, releases of to ...
Document
... A. the amount of product formed by a chemical reaction. B. whether or not a specific chemical reaction is possible. C. the coefficients needed to balance a chemical equation. ...
... A. the amount of product formed by a chemical reaction. B. whether or not a specific chemical reaction is possible. C. the coefficients needed to balance a chemical equation. ...
Chemical Reactions
... • We use chemical equations to summarize the process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as t ...
... • We use chemical equations to summarize the process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as t ...
ap chemistry – 2013-2014
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. This course is structured around six big ideas that include: Structure of matter, properties of matter-characteristics, states, and forces of attraction, chemical ...
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. This course is structured around six big ideas that include: Structure of matter, properties of matter-characteristics, states, and forces of attraction, chemical ...
Chemical Synthesis (sat6)
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
PowerPoint for Cornell Notes
... • Heartburn, as well as an acidic stomach due to eating too much spicy food, can be relieved by taking an antacid. The antacid is alkaline/basic and helps neutralize the stomach's acidic environment. If you look at the medicine for heartburn in the picture below, you may have used this one before to ...
... • Heartburn, as well as an acidic stomach due to eating too much spicy food, can be relieved by taking an antacid. The antacid is alkaline/basic and helps neutralize the stomach's acidic environment. If you look at the medicine for heartburn in the picture below, you may have used this one before to ...
Document
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
Faculty of Engineering and the Built Environment
... There are some 1600 companies in this sub-sector, employing around 45 000 staff. Plastics manufacturing is also a very diverse sub-sector and can be further broken down into several subindustries related to the raw material (input) and the manufacturing process. These are: • Plastics products and co ...
... There are some 1600 companies in this sub-sector, employing around 45 000 staff. Plastics manufacturing is also a very diverse sub-sector and can be further broken down into several subindustries related to the raw material (input) and the manufacturing process. These are: • Plastics products and co ...
Drug Testing - Uplift Grand
... What is actually measured in each of the three tests discussed? Spot color tests – looking for color change, indicates a chemical reaction has taken place IR spectrophotometry – measure absorbance of energy (light) by the bonds in a molecule GC-MS – measures the mass and charge of ions produced when ...
... What is actually measured in each of the three tests discussed? Spot color tests – looking for color change, indicates a chemical reaction has taken place IR spectrophotometry – measure absorbance of energy (light) by the bonds in a molecule GC-MS – measures the mass and charge of ions produced when ...
classification of chemical reactions
... Example: 2Na + Cl2 2NaCl Chemical change change in matter that produces new substances Example: rusting of iron burning of wood Physical change a change that does not produce a new substance a change in appearance or state Example: chopping wood ...
... Example: 2Na + Cl2 2NaCl Chemical change change in matter that produces new substances Example: rusting of iron burning of wood Physical change a change that does not produce a new substance a change in appearance or state Example: chopping wood ...
Balancing Chemical Equations
... • Relate the conservation of mass to the rearrangement of atoms in a chemical reaction • Write and interpret a balanced chemical equation for a reaction, and relate conservation of mass to the balanced equation ...
... • Relate the conservation of mass to the rearrangement of atoms in a chemical reaction • Write and interpret a balanced chemical equation for a reaction, and relate conservation of mass to the balanced equation ...
Matter_and_Change2
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
Transport of Material through Air, Soil, and Water
... investigate and evaluate potential risks resulting from consumer practices and industrial processes, and identify processes used in providing information and setting standards to manage these risks (e.g., interpret and explain the significance of manufacturer's information on how wood preservativ ...
... investigate and evaluate potential risks resulting from consumer practices and industrial processes, and identify processes used in providing information and setting standards to manage these risks (e.g., interpret and explain the significance of manufacturer's information on how wood preservativ ...
Estimating Mineral Weathering Rates in Catskills
... ◘ Basic Cations: Ca, Mg, K, Na ◘ Silica: H4SiO4 ◘ Aluminum: potentially toxic to aquatic biota ...
... ◘ Basic Cations: Ca, Mg, K, Na ◘ Silica: H4SiO4 ◘ Aluminum: potentially toxic to aquatic biota ...
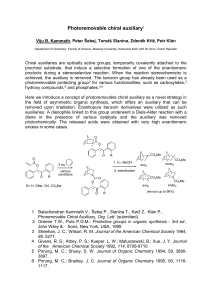
Viju B - IS MU
... products during a stereoselective reaction. When the reaction stereochemistry is achieved, the auxiliary is removed. The benzoin group has already been used as a photoremovable protecting group2 for various functionalities, such as carboxylates,3 hydroxy compounds,6 and phosphates.4,5 Here we introd ...
... products during a stereoselective reaction. When the reaction stereochemistry is achieved, the auxiliary is removed. The benzoin group has already been used as a photoremovable protecting group2 for various functionalities, such as carboxylates,3 hydroxy compounds,6 and phosphates.4,5 Here we introd ...
Chemical reactions unit
... RATES OF CHEMICAL REACTIONS There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so th ...
... RATES OF CHEMICAL REACTIONS There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so th ...
Chemical reactions unit
... RATES OF CHEMICAL REACTIONS There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so th ...
... RATES OF CHEMICAL REACTIONS There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so th ...
Chemical Reactions
... Decomposition …Separating Elements… A reactant breaks down into simpler products (opposite of synthesis) ...
... Decomposition …Separating Elements… A reactant breaks down into simpler products (opposite of synthesis) ...
Fine chemical

Fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg (see the comparison of fine chemicals, commodities and specialties). The class of fine chemicals is subdivided either on the basis of the added value (building blocks, advanced intermediates or active ingredients), or the type of business transaction, namely standard or exclusive products.Fine chemicals are produced in limited volumes (< 1000 tons/year) and at relatively high prices (> $10/kg) according to exacting specifications, mainly by traditional organic synthesis in multipurpose chemical plants. Biotechnical processes are gaining ground. The global production value is about $85 billion. Fine chemicals are used as starting materials for specialty chemicals, particularly pharmaceuticals, biopharmaceuticals and agrochemicals. Custom manufacturing for the life science industry plays a big role; however, a significant portion of the fine chemicals total production volume is manufactured in house by large users. The industry is fragmented and extends from small, privately owned companies to divisions of big, diversified chemical enterprises. The term ""fine chemicals"" is used in distinction to ""heavy chemicals"", which are produced and handled in large lots and are often in a crude state.Since their inception in the late 1970s, fine chemicals have become an important part of the chemical industry. The total production value of $85 billion is split about 60 / 40 among in-house production by the main consumers, the life science industry, on the one hand, and the fine chemicals industry on the other hand. The latter pursues both a “supply push” strategy, whereby standard products are developed in-house and offered ubiquitously, and a “demand pull” strategy, whereby products or services determined by the customer are provided exclusively on a “one customer / one supplier” basis. The products are mainly used as building blocks for proprietary products. The hardware of the top tier fine chemical companies has become almost identical. The design, lay-out and equipment of the plants and laboratories has become practically the same all over the world. Most chemical reactions performed go back to the days of the dyestuff industry. Numerous regulations determine the way labs and plants have to be operated, thereby contributing to the uniformity.