Calcium - IDC
... human and animal bones and dentin.[7] The mineral portion of some corals can also be transformed into hydroxylapatite. Calcium hydroxide (slaked lime), employed in numerous chemical refinery processes, is produced from applying heat to limestone at temperatures above 825 °C, following by hydration. ...
... human and animal bones and dentin.[7] The mineral portion of some corals can also be transformed into hydroxylapatite. Calcium hydroxide (slaked lime), employed in numerous chemical refinery processes, is produced from applying heat to limestone at temperatures above 825 °C, following by hydration. ...
Name: Date: AP Chemistry/Chemistry 145 Summer Assignment
... What mass of carbon dioxide gas is produced by the reaction of 0.250 moles of calcium carbonate? ...
... What mass of carbon dioxide gas is produced by the reaction of 0.250 moles of calcium carbonate? ...
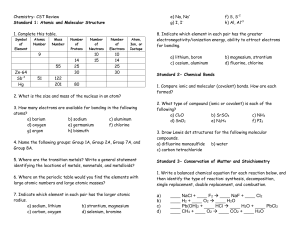
Chemistry- CST Review
... 6. How does changing the amount of gas, volume of gas, and temperature affect the gas pressure? For Q’s #9-14, name the gas law and show all your work. 7. The pressure on 2.00 L of anesthetic gas changes from 100 kPa to 40 kPa. What will be the new volume if the temperature remains constant? 8. If a ...
... 6. How does changing the amount of gas, volume of gas, and temperature affect the gas pressure? For Q’s #9-14, name the gas law and show all your work. 7. The pressure on 2.00 L of anesthetic gas changes from 100 kPa to 40 kPa. What will be the new volume if the temperature remains constant? 8. If a ...
CC-80 art 6
... The tricalcium silicate trihydrated derived from this reaction has extremely small particles and it forms a colloidal suspension. Calcium hydroxide (portlandite) is a crystalline solid. The hydration products of other cement components are not generally described as producing portlandite. If thermog ...
... The tricalcium silicate trihydrated derived from this reaction has extremely small particles and it forms a colloidal suspension. Calcium hydroxide (portlandite) is a crystalline solid. The hydration products of other cement components are not generally described as producing portlandite. If thermog ...
Biogeochemical Cycles
... >CO2 used to make sugars >CO2 used to make calcium carbonate >sugars broken down & releasing CO2 >dead organisms decomposing or turning into fossil fuels (coal, oil, natural gas) HUMAN INFLUENCE: >burning fossil fuels >clearing/burning forests & rainforests >mining fossils, minerals >planting crops, ...
... >CO2 used to make sugars >CO2 used to make calcium carbonate >sugars broken down & releasing CO2 >dead organisms decomposing or turning into fossil fuels (coal, oil, natural gas) HUMAN INFLUENCE: >burning fossil fuels >clearing/burning forests & rainforests >mining fossils, minerals >planting crops, ...
Too Hot to Handle Lab
... (rise/drop) _______________ in the temperature. If the (water/ bromo-blue) ________________________ is mixed with (NaHCO3 & CaCl2), the result will be (hydrogen/oxygen/carbon dioxide) ____________________ gas. The mixture of bromo-blue and ________________________ (NaHCO3 &/or CaCl2) will make the c ...
... (rise/drop) _______________ in the temperature. If the (water/ bromo-blue) ________________________ is mixed with (NaHCO3 & CaCl2), the result will be (hydrogen/oxygen/carbon dioxide) ____________________ gas. The mixture of bromo-blue and ________________________ (NaHCO3 &/or CaCl2) will make the c ...
C1 – Topic 2 notes - ARK Elvin Academy
... Calcium compounds can be used to neutralise soil acidity: Acids can be neutralised by alkalis. This is called a neutralisation reaction Some crops don’t grow well if the soil is too acidic to reduce acidity of the soil, farmers can spray alkalis such as calcium carbonate, calcium oxide or calcium o ...
... Calcium compounds can be used to neutralise soil acidity: Acids can be neutralised by alkalis. This is called a neutralisation reaction Some crops don’t grow well if the soil is too acidic to reduce acidity of the soil, farmers can spray alkalis such as calcium carbonate, calcium oxide or calcium o ...
Topic 2 notes - WordPress.com
... Calcium compounds can be used to neutralise soil acidity: Acids can be neutralised by alkalis. This is called a neutralisation reaction Some crops don’t grow well if the soil is too acidic to reduce acidity of the soil, farmers can spray alkalis such as calcium carbonate, calcium oxide or calcium o ...
... Calcium compounds can be used to neutralise soil acidity: Acids can be neutralised by alkalis. This is called a neutralisation reaction Some crops don’t grow well if the soil is too acidic to reduce acidity of the soil, farmers can spray alkalis such as calcium carbonate, calcium oxide or calcium o ...
DRILLING FLUID PRODUCTS - CALCIUM CHLORIDE PRODUCT
... Calcium Chloride is used as a soluble weighting agent for water base fluids. This product may also be used in oil based and synthetic drilling fluids. RECOMMENDED TREATMENT Calcium Chloride should be mixed through the hopper. Application rates will depend on drilling and fluid conditions. PRODUCT AD ...
... Calcium Chloride is used as a soluble weighting agent for water base fluids. This product may also be used in oil based and synthetic drilling fluids. RECOMMENDED TREATMENT Calcium Chloride should be mixed through the hopper. Application rates will depend on drilling and fluid conditions. PRODUCT AD ...
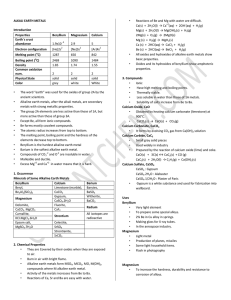
Chemical Names and Formulas
... silicon tetrabromide aluminium bromate Ca(HS)2 ammonium nitrite AgCH3COO calcium chromate N2O5 iron(III) carbonate HgS silver hydrogensulfate Copyright © 2004 McGraw-Hill Ryerson Limited. Permission to edit and reproduce this page is granted to the purchaser for use in her/his classroom. McGraw-Hill ...
... silicon tetrabromide aluminium bromate Ca(HS)2 ammonium nitrite AgCH3COO calcium chromate N2O5 iron(III) carbonate HgS silver hydrogensulfate Copyright © 2004 McGraw-Hill Ryerson Limited. Permission to edit and reproduce this page is granted to the purchaser for use in her/his classroom. McGraw-Hill ...
Chemical Equation Interpretations – Match the chemical equation
... carbonate in seashells and marble structures. This reaction produces calcium sulfate dissolved in water and carbon dioxide gas. ...
... carbonate in seashells and marble structures. This reaction produces calcium sulfate dissolved in water and carbon dioxide gas. ...
CHM121 Exam I Review
... reactant, product, mole, Avogadro's number, empirical formula, limiting reactant/reagent, theoretical yield, percent yield. 1. Complete the following conversion factors: 1 m = ______ cm ...
... reactant, product, mole, Avogadro's number, empirical formula, limiting reactant/reagent, theoretical yield, percent yield. 1. Complete the following conversion factors: 1 m = ______ cm ...
ALKALI EARTH METALS Introduction Properties Beryllium
... Ca (s) + 2HCI(aq) → CaCl2 + H2(g) Be (s) + 2HCI(aq) → BeCI2 + H2(g) All oxides and hydroxides of alkaline earth metals show basic properties. Oxides and its hydroxides of beryllium show amphoteric properties. ...
... Ca (s) + 2HCI(aq) → CaCl2 + H2(g) Be (s) + 2HCI(aq) → BeCI2 + H2(g) All oxides and hydroxides of alkaline earth metals show basic properties. Oxides and its hydroxides of beryllium show amphoteric properties. ...
Gas Stoichiometry Worksheet
... Gas Stoichiometry Practice For problems 1-3, assume that the reactions are being performed at a pressure of 1.0 atm and a temperature of 298 K. ...
... Gas Stoichiometry Practice For problems 1-3, assume that the reactions are being performed at a pressure of 1.0 atm and a temperature of 298 K. ...