anaerobic respiration
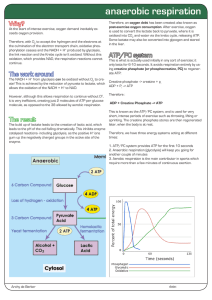
... The NADH + H+ from glycolysis can be oxidized without O2 to create! This is achieved by the reduction of pyruvate to lactate, which allows the oxidation of the NADH + H+ to NAD. However, although this allows respiration to continue without O2, it is very inefficient, creating just 2 molecules of ATP ...
... The NADH + H+ from glycolysis can be oxidized without O2 to create! This is achieved by the reduction of pyruvate to lactate, which allows the oxidation of the NADH + H+ to NAD. However, although this allows respiration to continue without O2, it is very inefficient, creating just 2 molecules of ATP ...
Name Date
... 16. The reactions of ___________ take place within the cytoplasm of eukaryotic cells. a. glycolysis c. chemiosmosis b. oxidation of pyruvate d. the electron transport chain ...
... 16. The reactions of ___________ take place within the cytoplasm of eukaryotic cells. a. glycolysis c. chemiosmosis b. oxidation of pyruvate d. the electron transport chain ...
Ecology - Winston Knoll Collegiate
... • Community (Biotic) interacting with environment (Abiotic ) ...
... • Community (Biotic) interacting with environment (Abiotic ) ...
What is an inference
... -Pyruvic Acid is broken down. -CO2 is produced - NAD+ and FAD+ accept electrons and become NADH and FADH2 ...
... -Pyruvic Acid is broken down. -CO2 is produced - NAD+ and FAD+ accept electrons and become NADH and FADH2 ...
Photosynthesis and Respiration
... Citric acid cycle (aka Krebs cycle) = similar to the Calvin cycle; produces CO2 and ATP Electron transport chain = energy released in steps to produce ATP and H2O ...
... Citric acid cycle (aka Krebs cycle) = similar to the Calvin cycle; produces CO2 and ATP Electron transport chain = energy released in steps to produce ATP and H2O ...
Human and Environment Lecture 4_7
... • Nitrogen fixing bacteria in the soil and in plant roots convert nitrogen from the atmosphere to ammonia. The ammonia is then used by plants or converted to nitrite and then nitrate by nitrifying bacteria. The nitrite and nitrate can also be used by plants. The plants are then eaten by herbivores, ...
... • Nitrogen fixing bacteria in the soil and in plant roots convert nitrogen from the atmosphere to ammonia. The ammonia is then used by plants or converted to nitrite and then nitrate by nitrifying bacteria. The nitrite and nitrate can also be used by plants. The plants are then eaten by herbivores, ...
EXTRA
... oxygen>21% O2). The process of energy production involves glycolysis, the Krebs’ cycle and the electron transport system for which O2 acts as a terminal electron acceptor. Energy is generated by the complete oxidation of the organic substrate to carbon dioxide and water. When glucose is the substrat ...
... oxygen>21% O2). The process of energy production involves glycolysis, the Krebs’ cycle and the electron transport system for which O2 acts as a terminal electron acceptor. Energy is generated by the complete oxidation of the organic substrate to carbon dioxide and water. When glucose is the substrat ...
Chapter 3 Notes
... Pigments- molecules that absorb specific colours of light Chlorophyll- a green pigment that absorbs all light but green light Why is this? light can be transmitted (pass through), reflected (bounce off) or absorbed a green substance reflects green light and either transmits or absorbs all other ...
... Pigments- molecules that absorb specific colours of light Chlorophyll- a green pigment that absorbs all light but green light Why is this? light can be transmitted (pass through), reflected (bounce off) or absorbed a green substance reflects green light and either transmits or absorbs all other ...
Cellular Respiration
... In most cells, not all of the carbon that enters glycolysis is converted to carbon dioxide by cellular respiration. What happens to this carbon that does not end up as CO2? (Concept 9.6 ...
... In most cells, not all of the carbon that enters glycolysis is converted to carbon dioxide by cellular respiration. What happens to this carbon that does not end up as CO2? (Concept 9.6 ...
Exam 2 Answers
... f. Involves the process of chemiosmosis. ETC g. A series of oxidation/reduction reactions that result in a proton gradient across the inner membrane of the mitochondria. ETC h. The means by which primitive organisms underwent cellular respiration in a pre-oxygen atmosphere Glycolysis ...
... f. Involves the process of chemiosmosis. ETC g. A series of oxidation/reduction reactions that result in a proton gradient across the inner membrane of the mitochondria. ETC h. The means by which primitive organisms underwent cellular respiration in a pre-oxygen atmosphere Glycolysis ...
notes - is234
... compounds in food to carry out their life activities. Almost all of the energy in carbon compounds comes from the sun. The sun is the ultimate source of energy for all living things. Plants, algae, and some prokaryotes carry out Photosynthesis, the process during which sunlight’s energy is used to c ...
... compounds in food to carry out their life activities. Almost all of the energy in carbon compounds comes from the sun. The sun is the ultimate source of energy for all living things. Plants, algae, and some prokaryotes carry out Photosynthesis, the process during which sunlight’s energy is used to c ...
chemotrophs
... • A heterotroph is a an organism that uses organic carbon for growth . This contrast with autotrophs , such plants which able to directly use source of energy , such as light to produce organic substrate from inorganic carbondioxide . • Herterotrophs are known as consumers in food chain . All animal ...
... • A heterotroph is a an organism that uses organic carbon for growth . This contrast with autotrophs , such plants which able to directly use source of energy , such as light to produce organic substrate from inorganic carbondioxide . • Herterotrophs are known as consumers in food chain . All animal ...
Cellular Respiration
... the same way under both aerobic (with oxygen) and anaerobic (without oxygen) conditions Splits apart a single glucose molecule (6 carbon) into two molecules of pyruvate (3 carbon). 2 ATP are yielded. Occurs in cytoplasm Under anaerobic conditions, pyruvate is converted by fermentation to lactic acid ...
... the same way under both aerobic (with oxygen) and anaerobic (without oxygen) conditions Splits apart a single glucose molecule (6 carbon) into two molecules of pyruvate (3 carbon). 2 ATP are yielded. Occurs in cytoplasm Under anaerobic conditions, pyruvate is converted by fermentation to lactic acid ...
A. glycolysis
... 1. oxidative phosphorylation – electrons are transferred from electron donors to electron acceptors such as oxygen – the energy released from this process is used to turn ADP into ATP – use of an electron transport chain (chemiosmosis) 2. substrate level phosphorylation – addition of a phosphate gro ...
... 1. oxidative phosphorylation – electrons are transferred from electron donors to electron acceptors such as oxygen – the energy released from this process is used to turn ADP into ATP – use of an electron transport chain (chemiosmosis) 2. substrate level phosphorylation – addition of a phosphate gro ...
Ch9 Review Sheet - Canvas by Instructure
... take place in these cells? Explain your answer. 20. How is the process by which your body extracts energy from food similar to how a car's engine extracts energy from fuel? How is it different? 21. Explain the following statement: Heterotrophs depend on autotrophs for energy. 22. What's Wrong With T ...
... take place in these cells? Explain your answer. 20. How is the process by which your body extracts energy from food similar to how a car's engine extracts energy from fuel? How is it different? 21. Explain the following statement: Heterotrophs depend on autotrophs for energy. 22. What's Wrong With T ...
E 5: Dissolved Oxygen in Water
... which contain sulfur, such as mercaptans. • Relatively active in low levels of BOD levels. ...
... which contain sulfur, such as mercaptans. • Relatively active in low levels of BOD levels. ...
1. Metabolism refers to A) pathways of chemical reactions that build
... 11. Electrons stripped from glucose during glycolysis and the Krebs cycle are transported to the electron transport chain by ... A) ADP. B) CO2. C) ATP. D) reduced coenzymes 12. At the end of the electron transport chain, the final acceptor of the electrons is ______, which produces a molecule of __ ...
... 11. Electrons stripped from glucose during glycolysis and the Krebs cycle are transported to the electron transport chain by ... A) ADP. B) CO2. C) ATP. D) reduced coenzymes 12. At the end of the electron transport chain, the final acceptor of the electrons is ______, which produces a molecule of __ ...
Examples of Lesson Plans
... of their digital cameras and how to transfer images from memory storage devices to their computers and then from their computers into a blog. A digital camera (many of the students will have phones which will accomplish this) ...
... of their digital cameras and how to transfer images from memory storage devices to their computers and then from their computers into a blog. A digital camera (many of the students will have phones which will accomplish this) ...
Microbial Metabolism
... Chemical energy is used to reduce CO2 to sugar (CH2O) Carbon Fixation - recycling of carbon in the environment (Life as we known is dependant on this) ...
... Chemical energy is used to reduce CO2 to sugar (CH2O) Carbon Fixation - recycling of carbon in the environment (Life as we known is dependant on this) ...
reactions --- electrons can`t flow in a vacuum, oxidation reactions
... DpH – pH differential across the membrane -- usually about 1.0 pH unit DY -- charge potential across the membrane (-160 mV) pmf - proton motive force (-240 mV for E. coli) DmH - proton activity (-32 kJ/mol) Uncouplers - ...
... DpH – pH differential across the membrane -- usually about 1.0 pH unit DY -- charge potential across the membrane (-160 mV) pmf - proton motive force (-240 mV for E. coli) DmH - proton activity (-32 kJ/mol) Uncouplers - ...
Cycles of Matter - MsHollandScience
... If it makes it way to oceans marine organisms can use it. Some phosphate stays on land and cycles between organisms and the soil. Plants absorb the phosphate from soil/water, plants bind phosphate into inorganic compounds. Organic phosphate moves through food web from producers to consumers and to t ...
... If it makes it way to oceans marine organisms can use it. Some phosphate stays on land and cycles between organisms and the soil. Plants absorb the phosphate from soil/water, plants bind phosphate into inorganic compounds. Organic phosphate moves through food web from producers to consumers and to t ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)























