Lecture 8 - Harford Community College
... generating ATP by phosphorylation, but uses inorganic molecule other than O2 , such as nitrate, as terminal electron acceptor • Anaerobic respiration produces less ATP than aerobic respiration ...
... generating ATP by phosphorylation, but uses inorganic molecule other than O2 , such as nitrate, as terminal electron acceptor • Anaerobic respiration produces less ATP than aerobic respiration ...
File
... •Energy is generated as electrons move through the electron transport chain, and is used to pump hydrogen ions (H+) from the matrix into the intermembrane space •This generates a concentration gradient which drives the hydrogen ions back into the matrix through enzymes called ATP Synthase. •ATP synt ...
... •Energy is generated as electrons move through the electron transport chain, and is used to pump hydrogen ions (H+) from the matrix into the intermembrane space •This generates a concentration gradient which drives the hydrogen ions back into the matrix through enzymes called ATP Synthase. •ATP synt ...
- Circle of Docs
... 39. Glutathione peroxidase is an enzyme in various redox reactions which serves to destroy peroxides and free radicals and requires which mineral as a cofactor? a. Zinc b. Selenium c. Iron d. Chromium ...
... 39. Glutathione peroxidase is an enzyme in various redox reactions which serves to destroy peroxides and free radicals and requires which mineral as a cofactor? a. Zinc b. Selenium c. Iron d. Chromium ...
Document
... 26. Meiotic division occurs mainly in sex cells to form gametes (sperms and ova)…………. 27. Starch is a storage polysaccharide stored in animal liver ………………………….......... 28. The viral capsid composed of protein units called capsomeres ....................................... 29. During cell respiratio ...
... 26. Meiotic division occurs mainly in sex cells to form gametes (sperms and ova)…………. 27. Starch is a storage polysaccharide stored in animal liver ………………………….......... 28. The viral capsid composed of protein units called capsomeres ....................................... 29. During cell respiratio ...
Photosynthesis
... The Equation Where do plants get carbon dioxide from? The atmosphere and our breathing Where do plants get water from? The soil, rain What do plants produce that we use? Oxygen (we breathe) and glucose (we eat) ...
... The Equation Where do plants get carbon dioxide from? The atmosphere and our breathing Where do plants get water from? The soil, rain What do plants produce that we use? Oxygen (we breathe) and glucose (we eat) ...
Notable Inventions - Lemelson
... were invisible during the imaging process, making it hard to recognize glycan structures that can report on diseases such as cancer. Bertozzi studies the relationship between glycosylation, the addition of sugar groups to a molecule, and disease – specifically how glycans contribute to bacterial inf ...
... were invisible during the imaging process, making it hard to recognize glycan structures that can report on diseases such as cancer. Bertozzi studies the relationship between glycosylation, the addition of sugar groups to a molecule, and disease – specifically how glycans contribute to bacterial inf ...
BSC1010 Quiz 2 Answers - Palm Beach State College
... A) energy released as electrons flow through the electron transport system B) energy released from substrate-level phosphorylation C) energy released from movement of protons through ATP synthase, down their electrochemical gradient D) No external source of energy is required because the reaction is ...
... A) energy released as electrons flow through the electron transport system B) energy released from substrate-level phosphorylation C) energy released from movement of protons through ATP synthase, down their electrochemical gradient D) No external source of energy is required because the reaction is ...
Physical and Chemical Changes
... smaller molecules called what? What type of compound is this? Carbohydrates are broken down into glucose molecules, which are a type of sugar. 2. Which element is found in abundance in the human body, but NOT found in abundance in the Earth? Carbon 3. What energy transformation is taking place when ...
... smaller molecules called what? What type of compound is this? Carbohydrates are broken down into glucose molecules, which are a type of sugar. 2. Which element is found in abundance in the human body, but NOT found in abundance in the Earth? Carbon 3. What energy transformation is taking place when ...
The Cell: A Microcosm of Life Multiple
... breaking covalent bonds). Examples include phosphorylation, carboxylation, glycosylation, or proenzyme activation by breaking a peptide bond. Mechanism 2: Allosteric regulation – here we also are not changing the abundance of the protein (in this case an enzyme), but we are inhibiting or stimulating ...
... breaking covalent bonds). Examples include phosphorylation, carboxylation, glycosylation, or proenzyme activation by breaking a peptide bond. Mechanism 2: Allosteric regulation – here we also are not changing the abundance of the protein (in this case an enzyme), but we are inhibiting or stimulating ...
Respiration - csfcA2Biology
... • ATP will release energy quickly, glucose will not, therefore it is an instant source of energy for the cell ...
... • ATP will release energy quickly, glucose will not, therefore it is an instant source of energy for the cell ...
Chapter Twenty-Seven: Amino Acids
... o Remove N-protection w dilute base o Rinse solid support o Add second N-protected amino acid via C-terminus ...
... o Remove N-protection w dilute base o Rinse solid support o Add second N-protected amino acid via C-terminus ...
Cellular Respiration Chapter 9
... The cell can use Fermentation instead!! Occurs in the Cytoplasm Just like glycolysis!! Fermentation A series of reactions that convert NADH (from glycolysis) back into NAD allowing glycolysis to keep producing a small amount of ATP ...
... The cell can use Fermentation instead!! Occurs in the Cytoplasm Just like glycolysis!! Fermentation A series of reactions that convert NADH (from glycolysis) back into NAD allowing glycolysis to keep producing a small amount of ATP ...
Document
... The electrons are extracted from the cofactors by reoxidation and then join the electron-transport chain, in this process, protons are expelled from the mitochondrion. The free energy stored in the resulting pH gradient drives the synthesis of ATP from ADP and Pi (inorganic phosphate) through oxida ...
... The electrons are extracted from the cofactors by reoxidation and then join the electron-transport chain, in this process, protons are expelled from the mitochondrion. The free energy stored in the resulting pH gradient drives the synthesis of ATP from ADP and Pi (inorganic phosphate) through oxida ...
File - Mr. Shanks` Class
... A chemical reaction involves the rearrangement of chemical bonds with the release or absorption of energy ...
... A chemical reaction involves the rearrangement of chemical bonds with the release or absorption of energy ...
II. Beta oxidation of fatty acid
... A. source of energy C. regulate hormone functions B. transport molecules D. preservation and transfer of genetic material _B__60. Complete hydrolysis of RNA nucleotides will yield this product, EXCEPT: A. phosphate C. adenine B. deoxyribose D. uracil _D__61. This type of mutation will result to a fr ...
... A. source of energy C. regulate hormone functions B. transport molecules D. preservation and transfer of genetic material _B__60. Complete hydrolysis of RNA nucleotides will yield this product, EXCEPT: A. phosphate C. adenine B. deoxyribose D. uracil _D__61. This type of mutation will result to a fr ...
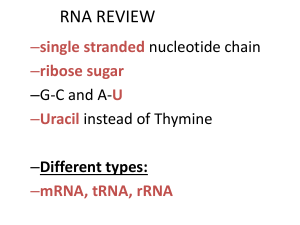
RNA and protein synthesis
... Proteins (polypeptides) are large polymers that are made from monomers called amino acids. Hundreds of amino acids linked together by peptide bonds and fold into a specific shape to make up a protein. There are 20 different types of amino acids. ...
... Proteins (polypeptides) are large polymers that are made from monomers called amino acids. Hundreds of amino acids linked together by peptide bonds and fold into a specific shape to make up a protein. There are 20 different types of amino acids. ...
Metabolic Pathways
... • Metabolic pathways are controlled by the presence or absence of particular enzymes in the metabolic pathway and through the regulation of the rate of reaction of key enzymes within the pathway. • Regulation can be controlled by intra- and extracellular signal molecules. • Induced fit and the role ...
... • Metabolic pathways are controlled by the presence or absence of particular enzymes in the metabolic pathway and through the regulation of the rate of reaction of key enzymes within the pathway. • Regulation can be controlled by intra- and extracellular signal molecules. • Induced fit and the role ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.