Biopolymers
... is that each base can only be paired with its “complementary” base: A with G, C with T. Note that the bases are joined by hydrogen bonds (discussed earlier). Base-pairing is the key to replication in DNA. Note that if it you could straighten out DNA, it would be about a mm (bacteria) to a cm (e.g. v ...
... is that each base can only be paired with its “complementary” base: A with G, C with T. Note that the bases are joined by hydrogen bonds (discussed earlier). Base-pairing is the key to replication in DNA. Note that if it you could straighten out DNA, it would be about a mm (bacteria) to a cm (e.g. v ...
chapter-6-rev - HCC Learning Web
... Why is it important to regenerate NAD+ molecules during fermentation? __________ is the only state in glucose metabolism that does not require oxygen to proceed. Two possible end products of fermentation are __________ as is produced by our muscle cell under anaerobic conditions and __________ by ye ...
... Why is it important to regenerate NAD+ molecules during fermentation? __________ is the only state in glucose metabolism that does not require oxygen to proceed. Two possible end products of fermentation are __________ as is produced by our muscle cell under anaerobic conditions and __________ by ye ...
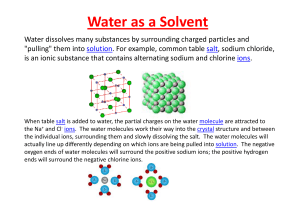
Water as a Solvent
... of chirality: The left hand is a non superposable mirror image of the right of chirality: The left hand is a non‐superposable mirror image of the right hand; no matter how the two hands are oriented, it is impossible for all the major features of both hands to coincide. This difference in symmetry ...
... of chirality: The left hand is a non superposable mirror image of the right of chirality: The left hand is a non‐superposable mirror image of the right hand; no matter how the two hands are oriented, it is impossible for all the major features of both hands to coincide. This difference in symmetry ...
Chapter 6, section 4 Topic: Enzymes Main concepts: •Proteins are
... so that the energy is useful to the cell and does not overheat the cell. • The structure of enzymes allows them to carry out reactions. Each enzyme is shaped to carry out only one specific reaction. This is known as enzyme specificity. Amylase, for example, is an enzyme that breaks down starch into ...
... so that the energy is useful to the cell and does not overheat the cell. • The structure of enzymes allows them to carry out reactions. Each enzyme is shaped to carry out only one specific reaction. This is known as enzyme specificity. Amylase, for example, is an enzyme that breaks down starch into ...
What minerals in trident gum make your mouth clean?
... Artificial flavorings so you do not know which one is actually in the product (Some can be VERY bad for you) ...
... Artificial flavorings so you do not know which one is actually in the product (Some can be VERY bad for you) ...
chapter8 - Teacherpage
... Cellular respiration evolved to enable organisms to utilize energy stored in glucose. Taps the energy found in the bonds of organic compounds (carbohydrates, lipids, proteins) ...
... Cellular respiration evolved to enable organisms to utilize energy stored in glucose. Taps the energy found in the bonds of organic compounds (carbohydrates, lipids, proteins) ...
Alkaline Phosphatase
... Bacterial phosphatase catalyzes the hydrolysis of phosphate esters, including those present in nucleic acids and nucleotides. Description: > More thermal stable than Calf Intestine Alkaline Phosphatase (CIAP, CIP). > Optimal incubation temperature is approximately 60oC, however the enzyme remains ac ...
... Bacterial phosphatase catalyzes the hydrolysis of phosphate esters, including those present in nucleic acids and nucleotides. Description: > More thermal stable than Calf Intestine Alkaline Phosphatase (CIAP, CIP). > Optimal incubation temperature is approximately 60oC, however the enzyme remains ac ...
CHM 20 EXAM 3 – REVIEW Name Ms Dang Indicate whether each
... 18. The hydrolysis of glycogen to yield glucose is catalyzed by the enzyme phospharylase. Caffeine, which is not a carbohydrate and not a substrate for the enzyme, inhibits phosphorylase. What kind of regulatory mechanism is at work? At first, one might expect the inhibition of phosphorylase action ...
... 18. The hydrolysis of glycogen to yield glucose is catalyzed by the enzyme phospharylase. Caffeine, which is not a carbohydrate and not a substrate for the enzyme, inhibits phosphorylase. What kind of regulatory mechanism is at work? At first, one might expect the inhibition of phosphorylase action ...
Photosynthesis and Respiration
... Production of cellular energy sources (ATP and NADH) for anaerobic and aerobic respiration. Production of pyruvate for use in the citric acid cycle. The production of intermediate carbon compounds, which can be removed for other cellular purposes. ...
... Production of cellular energy sources (ATP and NADH) for anaerobic and aerobic respiration. Production of pyruvate for use in the citric acid cycle. The production of intermediate carbon compounds, which can be removed for other cellular purposes. ...
Chapter 4: Amino Acids General Features of Amino Acids
... Peptide bond formation is a condensation reaction leading to the polymerization of amino acids into peptides and proteins. (Peptide: hormones, neurotransmitters, several antibiotics and antitumor agents) The presence of the carbonyl group in a peptide bond allows electron resonance stabilization to ...
... Peptide bond formation is a condensation reaction leading to the polymerization of amino acids into peptides and proteins. (Peptide: hormones, neurotransmitters, several antibiotics and antitumor agents) The presence of the carbonyl group in a peptide bond allows electron resonance stabilization to ...
1 - Temple College
... ü Determine which way water will move by osmosis and whether the cell will shrink, stay the same shape, or swell, when given the concentration of solutes in the cell and in the environment. Energy and Cells ü Explain the role of ATP (adenosine triphosphate) and ADP (adenosine diphosphate) in energ ...
... ü Determine which way water will move by osmosis and whether the cell will shrink, stay the same shape, or swell, when given the concentration of solutes in the cell and in the environment. Energy and Cells ü Explain the role of ATP (adenosine triphosphate) and ADP (adenosine diphosphate) in energ ...
Document
... so the molecules cannot pack together to form solids. • saturated fatty acids can pack together closely and can form solids. ...
... so the molecules cannot pack together to form solids. • saturated fatty acids can pack together closely and can form solids. ...
Chapter 7 - Coenzymes
... holoenzymes (active). There are two types of cofactors: 1) essential ions - metal ions -inorganic 2) coenzymes - organic molecules that act as group-transfer reagents (accept or donate groups)- can also be H+ and/or eBoth provide reactive groups not found on a.a. side chains. Coenzymes can be either ...
... holoenzymes (active). There are two types of cofactors: 1) essential ions - metal ions -inorganic 2) coenzymes - organic molecules that act as group-transfer reagents (accept or donate groups)- can also be H+ and/or eBoth provide reactive groups not found on a.a. side chains. Coenzymes can be either ...
cellular respiration
... • Cellular respiration can produce up to 38 ATP molecules for each glucose molecule consumed. • During cellular respiration, hydrogen and its bonding electrons change partners. – Hydrogen and its electrons go from sugar to oxygen, forming water. – This hydrogen transfer is why oxygen is so vital to ...
... • Cellular respiration can produce up to 38 ATP molecules for each glucose molecule consumed. • During cellular respiration, hydrogen and its bonding electrons change partners. – Hydrogen and its electrons go from sugar to oxygen, forming water. – This hydrogen transfer is why oxygen is so vital to ...
222 Coenzymes.p65
... This Factsheet summarises the role of coenzymes in photosynthesis and respiration and illustrates the types of exam questions which feature coenzymes. Coenzymes are small, organic, non-protein molecules that carry e.g. electrons and protons between enzymes. They are a type of cofactor – a substance ...
... This Factsheet summarises the role of coenzymes in photosynthesis and respiration and illustrates the types of exam questions which feature coenzymes. Coenzymes are small, organic, non-protein molecules that carry e.g. electrons and protons between enzymes. They are a type of cofactor – a substance ...
WHAT SHOULD I KNOW ABOUT RESPIRATION NAME ANSWERS
... What happens to pyruvate/pyruvic acid if there IS oxygen available? Moves into mitochondria and enters Krebs cycle What molecule acts as a helper to allow the carbons from pyruvate to enter the Krebs cycle? Co-enzyme A picks up carbons from pyruvic acid and becomes acetyl-CoA Which molecule forms fi ...
... What happens to pyruvate/pyruvic acid if there IS oxygen available? Moves into mitochondria and enters Krebs cycle What molecule acts as a helper to allow the carbons from pyruvate to enter the Krebs cycle? Co-enzyme A picks up carbons from pyruvic acid and becomes acetyl-CoA Which molecule forms fi ...
Chapter 3 Objectives
... of materials between the cell and its surroundings encloses all cells. Every cell, at some stage in its life, contains DNA, the heritable material that directs the cell’s many activities. All organisms are composed of cells. They occur singly as a great variety of unicellular organisms, and they occ ...
... of materials between the cell and its surroundings encloses all cells. Every cell, at some stage in its life, contains DNA, the heritable material that directs the cell’s many activities. All organisms are composed of cells. They occur singly as a great variety of unicellular organisms, and they occ ...
CHAPTER 39: The Genetic Code
... 73-93 ribonucleotides. L-shaped 3-D structure. Unusual bases. “Stems”: 3’-CCA acceptor stem, TΨC loop, DHU loop, and anticodon loop. 5. 5’-Phosphorylation. 6. Amino acid attached to 3’CCA. 7. Anticodon near center of sequence. ...
... 73-93 ribonucleotides. L-shaped 3-D structure. Unusual bases. “Stems”: 3’-CCA acceptor stem, TΨC loop, DHU loop, and anticodon loop. 5. 5’-Phosphorylation. 6. Amino acid attached to 3’CCA. 7. Anticodon near center of sequence. ...
Energy Systems - margolis sport exercise
... iii. Electron transport chain: is used to transport electrons from NADH and FADH2. *H+ pumped across the membrane diffuse back in to generate 32 ATP (oxidative phosphorylation). *Oxygen acts as the final H+ acceptor to form H2O. *occurs at a rate of 10,000,000 ATP per/sec in working muscle cells! ...
... iii. Electron transport chain: is used to transport electrons from NADH and FADH2. *H+ pumped across the membrane diffuse back in to generate 32 ATP (oxidative phosphorylation). *Oxygen acts as the final H+ acceptor to form H2O. *occurs at a rate of 10,000,000 ATP per/sec in working muscle cells! ...
I. Cellular Energy • ATP: a) When the terminal phosphate is removed
... a) Aerobic Respiration: occurs in the presence of O2 & is capable of producing large quantities of ATP. Practiced by some unicellular & ALL multicellular organisms. b) Anaerobic Respiration: does not utilize oxygen, but rather some other electronegative atom or molecule to drive ATP synthesis. Is no ...
... a) Aerobic Respiration: occurs in the presence of O2 & is capable of producing large quantities of ATP. Practiced by some unicellular & ALL multicellular organisms. b) Anaerobic Respiration: does not utilize oxygen, but rather some other electronegative atom or molecule to drive ATP synthesis. Is no ...
Energy Production - University of Massachusetts Amherst
... breakdown of protein – mostly from lean muscle) provides energy for biologic work. • VISA: using protein as energy supplements the ATP/PCr, glycogen and fatty acids that provide the majoroty of the ATP. ...
... breakdown of protein – mostly from lean muscle) provides energy for biologic work. • VISA: using protein as energy supplements the ATP/PCr, glycogen and fatty acids that provide the majoroty of the ATP. ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.