complex I
... The human mitochondrial genome contains about 16500 nucleotides and encodes 2 ribosomal RNAs, 22 transfer RNAs, and 13 different polypeptide chains. ...
... The human mitochondrial genome contains about 16500 nucleotides and encodes 2 ribosomal RNAs, 22 transfer RNAs, and 13 different polypeptide chains. ...
Protein: Amino Acids
... • After reading Chapter 5, class discussion and activities you will be able to: – Describe the role of proteins – Distinguish between complete and incomplete proteins – Identify sources of quality protein – Calculate calories from protein ...
... • After reading Chapter 5, class discussion and activities you will be able to: – Describe the role of proteins – Distinguish between complete and incomplete proteins – Identify sources of quality protein – Calculate calories from protein ...
Chapter 8 Notes
... The release of energy during the hydrolysis of ATP comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves. Why does the hydrolysis of ATP yield so much energy? o Each of the three phosphate groups has a negative charge. o These three like charges are ...
... The release of energy during the hydrolysis of ATP comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves. Why does the hydrolysis of ATP yield so much energy? o Each of the three phosphate groups has a negative charge. o These three like charges are ...
Intermediary Metabolism Intermediary Metabolism
... acetyl CoA carboxylase (transcription) fatty acid synthase ...
... acetyl CoA carboxylase (transcription) fatty acid synthase ...
Oxidative phosphorylation RESP312
... The transport of a pair of electrons from NADH (and FMNH2) to oxygen via the electron transport chain produces energy which is more than sufficient to produce 3 ATPs from 3 ADP and 3 Pi. The transport of a pair of electrons from FADH2 to oxygen via the ETC produces sufficient energy to produce 2 ATP ...
... The transport of a pair of electrons from NADH (and FMNH2) to oxygen via the electron transport chain produces energy which is more than sufficient to produce 3 ATPs from 3 ADP and 3 Pi. The transport of a pair of electrons from FADH2 to oxygen via the ETC produces sufficient energy to produce 2 ATP ...
MNV-VPg-eIF4G-paper.SuppInfo.v2 07/08/2015 A conserved
... mM 2-mercaptoethanol, two of the mutant proteins – 116DYGE116RAPK and WADD108APRR – were found to have OD260/280 ratios of 1.51 and 1.95 respectively. ...
... mM 2-mercaptoethanol, two of the mutant proteins – 116DYGE116RAPK and WADD108APRR – were found to have OD260/280 ratios of 1.51 and 1.95 respectively. ...
Lecture 29
... Now substrate is bound to DnaK (loosely), Binding of DnaJ accelerates the ATPase activity by I 0” 3) ATP is hydrolyzed to ADP and DnaJ dissociates, the substrate is now tightly bound (very stable) in absence of NEF (GrpE) this complex can be stable for 20 s to minutes, sufficient to translate a prot ...
... Now substrate is bound to DnaK (loosely), Binding of DnaJ accelerates the ATPase activity by I 0” 3) ATP is hydrolyzed to ADP and DnaJ dissociates, the substrate is now tightly bound (very stable) in absence of NEF (GrpE) this complex can be stable for 20 s to minutes, sufficient to translate a prot ...
Small Business Success on the Web
... Important cell component animal cell membranes precursor of all other steroids ...
... Important cell component animal cell membranes precursor of all other steroids ...
6 Section B Exercise and Sport Physiology (Option B3) 5 (a
... anaerobic glycolysis, 2 in Krebs cycle/34 in the electron transport chain no fatiguing by products are produced/carbon dioxide & water easily removed able to work for long periods of time fats can also be used / provide more energy fat can also be used as a fuel ...
... anaerobic glycolysis, 2 in Krebs cycle/34 in the electron transport chain no fatiguing by products are produced/carbon dioxide & water easily removed able to work for long periods of time fats can also be used / provide more energy fat can also be used as a fuel ...
Small Business Success on the Web
... Important cell component animal cell membranes precursor of all other steroids ...
... Important cell component animal cell membranes precursor of all other steroids ...
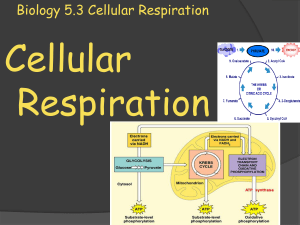
Biology 5.3 Cellular Respiration
... waste products of respiration. A simple formula to show respiration looks like this: Glucose + oxygen carbon dioxide (waste) + water (waste) + energy ...
... waste products of respiration. A simple formula to show respiration looks like this: Glucose + oxygen carbon dioxide (waste) + water (waste) + energy ...
chapter_14_respiration_in_plants
... ETS or electron transport system is located in the inner mitochondrial membrane. It helps in releasing and utilizing the energy stored in NADH+H+ and FADH2. NADH + H+, which is formed during glycolysis and citric acid cycle, gets oxidized by NADH dehydrogenase (complex I). The electrons so generated ...
... ETS or electron transport system is located in the inner mitochondrial membrane. It helps in releasing and utilizing the energy stored in NADH+H+ and FADH2. NADH + H+, which is formed during glycolysis and citric acid cycle, gets oxidized by NADH dehydrogenase (complex I). The electrons so generated ...
Instructions for Biochemistry
... are 4 possible bases at three different positions, there are 4 X 4 X 4 = 64 possible codons, which are more than enough for the 20 amino acids. There are also specific codons to signal the beginning and the end of an amino acid chain. Proteins are extremely varied in size and function, and this vari ...
... are 4 possible bases at three different positions, there are 4 X 4 X 4 = 64 possible codons, which are more than enough for the 20 amino acids. There are also specific codons to signal the beginning and the end of an amino acid chain. Proteins are extremely varied in size and function, and this vari ...
Amino Acid Synthesis
... b. There are 10 essential amino acids we cannot make. c. Looking back in evolution, why is it that bugs can make all these and we can’t? It turns out that one thing you can look at is how badly we need them and how difficult it is to make them. d. We do not need any of them because we cannot get the ...
... b. There are 10 essential amino acids we cannot make. c. Looking back in evolution, why is it that bugs can make all these and we can’t? It turns out that one thing you can look at is how badly we need them and how difficult it is to make them. d. We do not need any of them because we cannot get the ...
n-formyl methionine
... N-Formylmethionine Formylmethionine (fMet) is an amino acid found in all living cells. It is a derivative of the amino acid methionine. It is a modified form of methionine in which a formyl group has been added to methionine's amino group. It plays a crucial part in the protein synthesis of bacteria ...
... N-Formylmethionine Formylmethionine (fMet) is an amino acid found in all living cells. It is a derivative of the amino acid methionine. It is a modified form of methionine in which a formyl group has been added to methionine's amino group. It plays a crucial part in the protein synthesis of bacteria ...
+ energy
... proton) from an oxidizable substrate. The hydride ion is added to either the front (the A side) or the back (the B side) of the planar nicotinamide ring. (b) The UV absorption spectra of NAD+ and NADH. Reduction of the nicotinamide ring produces a new, broad absorption band with a maximum at 340 nm. ...
... proton) from an oxidizable substrate. The hydride ion is added to either the front (the A side) or the back (the B side) of the planar nicotinamide ring. (b) The UV absorption spectra of NAD+ and NADH. Reduction of the nicotinamide ring produces a new, broad absorption band with a maximum at 340 nm. ...
electron transport chain
... pathways to power the biosynthesis of amino acids, fats, and sugars to provide virtually all the heat needed to maintain body temperature to create energy sources, such as glucose or amino acids, that are recycled back through respiration, thus allowing a continual source of ATP with relatively litt ...
... pathways to power the biosynthesis of amino acids, fats, and sugars to provide virtually all the heat needed to maintain body temperature to create energy sources, such as glucose or amino acids, that are recycled back through respiration, thus allowing a continual source of ATP with relatively litt ...
Hücrede Enerji Metabolizması
... •Conversion of 3phosphoglycerate to glucose is very similar to gluconeogenesis, but glyceraldehyde dehydrogenase uses NADPH not NADH. •Steps require consumption of ATP and NADPH. •3-phosphoglycerate could also be exported to cytsol and be used in normal gluconeogenesis. •Hexoses can then be used for ...
... •Conversion of 3phosphoglycerate to glucose is very similar to gluconeogenesis, but glyceraldehyde dehydrogenase uses NADPH not NADH. •Steps require consumption of ATP and NADPH. •3-phosphoglycerate could also be exported to cytsol and be used in normal gluconeogenesis. •Hexoses can then be used for ...
Chapter 1
... • The functional form of many proteins is not that of a single polypeptide chain, but actually an aggregate of several globular peptides • Quaternary structure: the arrangement of subunits or peptides that form a larger protein • Subunit: a polypeptide chain having primary, secondary, and tertiary s ...
... • The functional form of many proteins is not that of a single polypeptide chain, but actually an aggregate of several globular peptides • Quaternary structure: the arrangement of subunits or peptides that form a larger protein • Subunit: a polypeptide chain having primary, secondary, and tertiary s ...
From Amino Acids to Proteins - in 4 Easy Steps
... A. Hydrophobic amino acids are buried in the interior of a globular protein. • Hydrophobic amino acids are composed primarily of carbon atoms, which cannot form hydrogen bonds with water. In order to form a hydrogen bond with water, a polar molecule, the amino acid side chains must also be polar, o ...
... A. Hydrophobic amino acids are buried in the interior of a globular protein. • Hydrophobic amino acids are composed primarily of carbon atoms, which cannot form hydrogen bonds with water. In order to form a hydrogen bond with water, a polar molecule, the amino acid side chains must also be polar, o ...
Enzymology Lecture 5 - ASAB-NUST
... Prosthetic groups, which are tightly bound to an enzyme, or coenzymes, which are released from the enzyme's active site during the reaction. Coenzymes include NADH, NADPH and adenosine triphosphate . These molecules act to transfer chemical groups between enzymes. carbonic anhydrase, with a zinc cof ...
... Prosthetic groups, which are tightly bound to an enzyme, or coenzymes, which are released from the enzyme's active site during the reaction. Coenzymes include NADH, NADPH and adenosine triphosphate . These molecules act to transfer chemical groups between enzymes. carbonic anhydrase, with a zinc cof ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.