Document
... • Cytochrome oxidase catalyzes the reduction of a final electron acceptor, oxygen • An artifcial e- donor, phenylenediamine, is used to reduce the cytochrome oxidase • If the enzyme is present, the colorless reagent (reduced state) will turn blue (oxidized state) ...
... • Cytochrome oxidase catalyzes the reduction of a final electron acceptor, oxygen • An artifcial e- donor, phenylenediamine, is used to reduce the cytochrome oxidase • If the enzyme is present, the colorless reagent (reduced state) will turn blue (oxidized state) ...
Amino Acids, Amino Sugars and Sugars Present in
... Wiley & Wilson, 1961); strains AED (type 12 T antigen), GL 8 (type 19 M), ADA (type 14 M) obtained from Dr L. Dienes (Boston U.S.A.) and strains B6 (typing pattern 11/27/44) isolated locally in Leiden, Netherlands, As controls, two strains of group C streptococci, one of human origin and carrying a ...
... Wiley & Wilson, 1961); strains AED (type 12 T antigen), GL 8 (type 19 M), ADA (type 14 M) obtained from Dr L. Dienes (Boston U.S.A.) and strains B6 (typing pattern 11/27/44) isolated locally in Leiden, Netherlands, As controls, two strains of group C streptococci, one of human origin and carrying a ...
Converting Sugars to Biofuels: Ethanol and Beyond
... LCB-derived ethanol. Since cellular processes involved in sugar consumption are complex, more systematic engineering of various factors such as transporters, regulatory mechanisms of catabolites and cellular responses to stress caused by intermediates and products would be required. Improved sugar u ...
... LCB-derived ethanol. Since cellular processes involved in sugar consumption are complex, more systematic engineering of various factors such as transporters, regulatory mechanisms of catabolites and cellular responses to stress caused by intermediates and products would be required. Improved sugar u ...
Teacher Key - Free-2
... Model Worksheet and Summary Table your classroom. Have your students rotate through each station to explore the models and record their observations on the Model Worksheet and Summary Table. ...
... Model Worksheet and Summary Table your classroom. Have your students rotate through each station to explore the models and record their observations on the Model Worksheet and Summary Table. ...
ppt - Manning`s Science
... specificity of substrate biding, but the human body could not function if all enzymes were present together and all operating maximally with no regulation. ...
... specificity of substrate biding, but the human body could not function if all enzymes were present together and all operating maximally with no regulation. ...
as Powerpoint presentation
... reactions leads to movement of protons from inside to the outside, producing protonmotive force (electrochemical gradient of protons) that is used to drive protons back in by way of the ATP synthase, providing the energy for phosphorylation. The oxidation of one molecule of NADH is coupled to (appro ...
... reactions leads to movement of protons from inside to the outside, producing protonmotive force (electrochemical gradient of protons) that is used to drive protons back in by way of the ATP synthase, providing the energy for phosphorylation. The oxidation of one molecule of NADH is coupled to (appro ...
Bis2A 07.2 Fermentation
... In glycolysis, NAD+ is converted to NADH; what happens to the NADH produced? During glycolysis NAD+ is reduced to NADH and glucose is oxidized to pyruvate. During this process the cells must regenerate NAD+ by a second redox reaction. In respiration, this occurs when NADH is used ...
... In glycolysis, NAD+ is converted to NADH; what happens to the NADH produced? During glycolysis NAD+ is reduced to NADH and glucose is oxidized to pyruvate. During this process the cells must regenerate NAD+ by a second redox reaction. In respiration, this occurs when NADH is used ...
Section 2 Types of Chemical Reactions Chapter 8
... FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) ...
... FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) ...
Poster
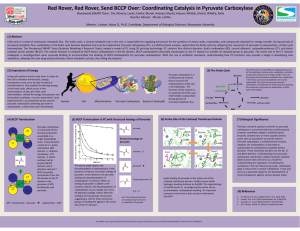
... Cells exist in a state of continuous metabolic flux. The Krebs cycle, a central metabolic hub in the cell, is responsible for supplying precursors for the synthesis of amino acids, nucleotides, and compounds required for energy transfer. During periods of increased metabolic flux, metabolites in the ...
... Cells exist in a state of continuous metabolic flux. The Krebs cycle, a central metabolic hub in the cell, is responsible for supplying precursors for the synthesis of amino acids, nucleotides, and compounds required for energy transfer. During periods of increased metabolic flux, metabolites in the ...
Chapter 9. Cellular Respiration STAGE 1: Glycolysis
... transfer energy from organic molecules to ATP still is starting point for ALL cellular respiration ...
... transfer energy from organic molecules to ATP still is starting point for ALL cellular respiration ...
Chapter 9. Cellular Respiration STAGE 1: Glycolysis
... transfer energy from organic molecules to ATP still is starting point for ALL cellular respiration ...
... transfer energy from organic molecules to ATP still is starting point for ALL cellular respiration ...
Units of Competency
... workload being met at a certain HR and BR. When an exercising subject goes from rest to exercise their physiological systems cannot reach steady state immediately because there is a lag time when the HR and BR need to ...
... workload being met at a certain HR and BR. When an exercising subject goes from rest to exercise their physiological systems cannot reach steady state immediately because there is a lag time when the HR and BR need to ...
Materials and Methods S1.
... synthesis was monitored during 4-15 min at 37 °C. ATP was extracted by rapidly boiling the cells suspension for 10 min. Cell debris were removed by centrifugation (20000 x g, for 15 min, 4 °C) and the ATP crude extract (ATPce) obtained was cooled on ice and used for ATP evaluation. Light emission wa ...
... synthesis was monitored during 4-15 min at 37 °C. ATP was extracted by rapidly boiling the cells suspension for 10 min. Cell debris were removed by centrifugation (20000 x g, for 15 min, 4 °C) and the ATP crude extract (ATPce) obtained was cooled on ice and used for ATP evaluation. Light emission wa ...
INSIDER`S GUIDE Interpretation and treatment: Organic acid
... The citric acid cycle is the all important metabolic pathway which uses a series of enzymecatalysed chemical reactions involved in the conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. Other relevant reactions in the pathway include tho ...
... The citric acid cycle is the all important metabolic pathway which uses a series of enzymecatalysed chemical reactions involved in the conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. Other relevant reactions in the pathway include tho ...
Muscle Energy Metabolism
... three mechanisms of ATP resynthesis are through anaerobic metabolism, which means without the use of oxygen. Anaerobic energy metabolism, also called anaerobic glycolysis, involves the incomplete breakdown of carbohydrates to lactic acid via anaerobic metabolic pathways. Anaerobic glycolysis is invo ...
... three mechanisms of ATP resynthesis are through anaerobic metabolism, which means without the use of oxygen. Anaerobic energy metabolism, also called anaerobic glycolysis, involves the incomplete breakdown of carbohydrates to lactic acid via anaerobic metabolic pathways. Anaerobic glycolysis is invo ...
Chapter 9 Proteins - Angelo State University
... of polypeptides joined by peptide bonds between the amino and carboxyl groups of amino acid residues. • Proteins perform a number of vital functions: – Enzymes are proteins that act as biochemical catalysts. – Many proteins have structural or mechanical functions (e.g., actin and myosin in muscles). ...
... of polypeptides joined by peptide bonds between the amino and carboxyl groups of amino acid residues. • Proteins perform a number of vital functions: – Enzymes are proteins that act as biochemical catalysts. – Many proteins have structural or mechanical functions (e.g., actin and myosin in muscles). ...
Organic Chemistry
... Maitotoxin, a complex organic biological toxin Biomolecular chemistry is a major category within organic chemistry which is frequently studied by biochemists. Many complex multi-functional group molecules are important in living organisms. Some are long-chain biopolymers, and these include peptides, ...
... Maitotoxin, a complex organic biological toxin Biomolecular chemistry is a major category within organic chemistry which is frequently studied by biochemists. Many complex multi-functional group molecules are important in living organisms. Some are long-chain biopolymers, and these include peptides, ...
Enzymes - HCC Learning Web
... The Process of Science: Can Enzymes Be Engineered? • Observation: Genetic sequences suggest that many of our genes were formed through a type of molecular evolution. • Question: Can laboratory methods form new enzymes through ...
... The Process of Science: Can Enzymes Be Engineered? • Observation: Genetic sequences suggest that many of our genes were formed through a type of molecular evolution. • Question: Can laboratory methods form new enzymes through ...
Ch. 9: Cellular Respiration
... citric acid cycle Fats digested to glycerol used in glycolysis; fatty acids used in generating acetyl CoA) Fatty acids broken down by beta oxidation yield acetyl CoA ...
... citric acid cycle Fats digested to glycerol used in glycolysis; fatty acids used in generating acetyl CoA) Fatty acids broken down by beta oxidation yield acetyl CoA ...
Chapter 4 The Three-Dimensional Structure of Proteins
... 17. How can changes in pH alter the conformation of a protein? Answer: Changes in pH can influence the extent to which certain amino acid side chains (or the amino and carboxyl termini) are protonated. The result is a change in net charge on the protein, which can lead to electrostatic attractions o ...
... 17. How can changes in pH alter the conformation of a protein? Answer: Changes in pH can influence the extent to which certain amino acid side chains (or the amino and carboxyl termini) are protonated. The result is a change in net charge on the protein, which can lead to electrostatic attractions o ...
All Proteins Have a Basic Molecular Formula
... classifications encompass many processes and elements, ranging from pathways to cellular compartments. These functional classifications have been shown to overlap considerably with each other [4]. Functionally, proteins are classified into the following: enzymes (proteins that catalyze chemical and ...
... classifications encompass many processes and elements, ranging from pathways to cellular compartments. These functional classifications have been shown to overlap considerably with each other [4]. Functionally, proteins are classified into the following: enzymes (proteins that catalyze chemical and ...
Transferase-catalyses transfer of a group from one molecule to
... organic or metalloorganic molecule called a coenzyme. Some enzymes require both a coenzyme and one or more metal ions for activity. A cofactor or coenzyme that is covalently bound to the enzyme is called a prosthetic group. A complete, catalytically active enzyme together with its coenzyme and/or me ...
... organic or metalloorganic molecule called a coenzyme. Some enzymes require both a coenzyme and one or more metal ions for activity. A cofactor or coenzyme that is covalently bound to the enzyme is called a prosthetic group. A complete, catalytically active enzyme together with its coenzyme and/or me ...
103 Lecture Ch21a
... glucose and fructose can be written as follows: E + S ES E + P1 + P2 where E = sucrase, S = sucrose, P1 = glucose and P2 = fructose ...
... glucose and fructose can be written as follows: E + S ES E + P1 + P2 where E = sucrase, S = sucrose, P1 = glucose and P2 = fructose ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.