國立嘉義大學九十二學年度
... (A) The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature. (B) The molecules of an ideal gas are relatively far apart. (C) All molecules of an ideal gas have the same kinetic energy at constant temperature. (D) Molecules of a gas underg ...
... (A) The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature. (B) The molecules of an ideal gas are relatively far apart. (C) All molecules of an ideal gas have the same kinetic energy at constant temperature. (D) Molecules of a gas underg ...
Equilibrium Constant
... Kinetics tells us if the system will actually achieve this state within a reasonable time. ...
... Kinetics tells us if the system will actually achieve this state within a reasonable time. ...
Rapid Microwave Synthesis, Characterization and Reactivity
... The DTA profile for sample 8 however reveals an interesting feature above 700 K with no corresponding simultaneous weight change. This endothermic peak at 770 K can thus be attributed to a structural phase transition in Li4NH. An equivalent exothermic peak in the DTA was observed at 755.6 K on cooli ...
... The DTA profile for sample 8 however reveals an interesting feature above 700 K with no corresponding simultaneous weight change. This endothermic peak at 770 K can thus be attributed to a structural phase transition in Li4NH. An equivalent exothermic peak in the DTA was observed at 755.6 K on cooli ...
6. Thermodynamics - Sakshi Education
... equilibrium temperature (T) during the transformation. The units of ∆S are J mol–1 K–1. Entropy is a state function and is an extensive property. Ex: Ice ⇌ Water ⇌ Vapour .The order of entropy is S(g) > S(l) > S(s) Entropy increases in all spontaneous processes 6. State second law of Thermodynamics. ...
... equilibrium temperature (T) during the transformation. The units of ∆S are J mol–1 K–1. Entropy is a state function and is an extensive property. Ex: Ice ⇌ Water ⇌ Vapour .The order of entropy is S(g) > S(l) > S(s) Entropy increases in all spontaneous processes 6. State second law of Thermodynamics. ...
Reversible and irreversible reactions - Chemwiki
... In this case also some amount of gaseous hydrogen iodide will be left unreacted. This means that the products of certain reactions can be converted back to the reactants. These types of reactions are called reversible reactions. Thus, in reversible reactions the products can react with one another u ...
... In this case also some amount of gaseous hydrogen iodide will be left unreacted. This means that the products of certain reactions can be converted back to the reactants. These types of reactions are called reversible reactions. Thus, in reversible reactions the products can react with one another u ...
File
... 59. The ionization constant, Kb, of the base HONH2 is 1.1 x 108 . The pH of a 1.0 M aqueous soluton of HONH2 is closest to A) 4.0 B) 6.0 C) 8.0 D) 10.0 E) 14.0 60. Which of the following 0.10 M aqueous solutions has a pH less than 7 ? A) KI B) NH4NO3 C) K2CO3 D) NH3 E) Ca(OH)2 61. If 0.15 mol of K2 ...
... 59. The ionization constant, Kb, of the base HONH2 is 1.1 x 108 . The pH of a 1.0 M aqueous soluton of HONH2 is closest to A) 4.0 B) 6.0 C) 8.0 D) 10.0 E) 14.0 60. Which of the following 0.10 M aqueous solutions has a pH less than 7 ? A) KI B) NH4NO3 C) K2CO3 D) NH3 E) Ca(OH)2 61. If 0.15 mol of K2 ...
Making Connections - SCH4U1-CCVI
... the greater the # of ways particles can arrange themselves, the less ordered they are the greater the # of ways a particular state can be achieved, the more likely that state is going to exist Entropy , Sº The measure of disorder or randomness increased disorder or entropy favours spontaneit ...
... the greater the # of ways particles can arrange themselves, the less ordered they are the greater the # of ways a particular state can be achieved, the more likely that state is going to exist Entropy , Sº The measure of disorder or randomness increased disorder or entropy favours spontaneit ...
UNIT 1 - StudyGuide.PK
... area under them, the maximum of the higher temperature curve will have a lower y-axis value. For a given Eact, there is a larger number of molecules with E ⇒ Eact at the higher temperature. Heterogeneous catalysts often work by weakening the bonds in the reactants by the process of adsorption. Homog ...
... area under them, the maximum of the higher temperature curve will have a lower y-axis value. For a given Eact, there is a larger number of molecules with E ⇒ Eact at the higher temperature. Heterogeneous catalysts often work by weakening the bonds in the reactants by the process of adsorption. Homog ...
Chapter 1 Matter and Change
... - There are two categories of pure substances: Elements and Compounds ...
... - There are two categories of pure substances: Elements and Compounds ...
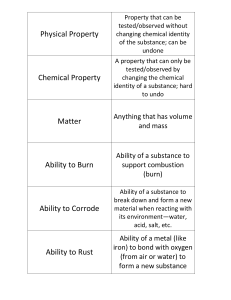
Physical Property
... Ability of a metal (like iron) to bond with oxygen (from air or water) to form a new substance ...
... Ability of a metal (like iron) to bond with oxygen (from air or water) to form a new substance ...
Exam 2-f06 - Clayton State University
... 2.) Which one of the following statements is false? a.) In order for a reaction to occur, reactant molecules must collide with each other. b.) A catalyst alters the rate of a reaction and is neither a product nor a reactant in the overall equation. c.) According to collision theory a three body coll ...
... 2.) Which one of the following statements is false? a.) In order for a reaction to occur, reactant molecules must collide with each other. b.) A catalyst alters the rate of a reaction and is neither a product nor a reactant in the overall equation. c.) According to collision theory a three body coll ...
First Law of Thermodynamics
... obvious examples. The intensive property of density can quickly be derived. For an ideal gas, the molar density d equals p/RT and hence is independent of the amount of material. Extensive variable. Some quantities such as volume are directly proportional to the amount of material. They are extensive ...
... obvious examples. The intensive property of density can quickly be derived. For an ideal gas, the molar density d equals p/RT and hence is independent of the amount of material. Extensive variable. Some quantities such as volume are directly proportional to the amount of material. They are extensive ...
semester two review sheet
... 4. Why is water considered the universal solvent? 5. What are the special properties of water and why do they occur? 6. Explain why solid ice is less than liquid water with regard to particle arrangement. 7. Why does a substance like sugar dissolve in water, but oil does not? SOLUTIONS 1. Define the ...
... 4. Why is water considered the universal solvent? 5. What are the special properties of water and why do they occur? 6. Explain why solid ice is less than liquid water with regard to particle arrangement. 7. Why does a substance like sugar dissolve in water, but oil does not? SOLUTIONS 1. Define the ...
Pages from PS 11 Textbook for Lab
... calorimeter, for which ΔU = ΔU therm + ΔU chem = q + w = qV where w = –p∆V = 0 because the rigid walls of the bomb prevented any change in volume, ∆V = 0. So the release of chemical energy upon combustion appeared as an increase in thermal energy at constant volume, qV. However, a great many chemica ...
... calorimeter, for which ΔU = ΔU therm + ΔU chem = q + w = qV where w = –p∆V = 0 because the rigid walls of the bomb prevented any change in volume, ∆V = 0. So the release of chemical energy upon combustion appeared as an increase in thermal energy at constant volume, qV. However, a great many chemica ...
File
... c. neither a nor b b. chemical equilibrium. d. both a and b 8. According to collision theory, in order for a chemical reaction to occur, the reactant atoms must: a. make contact with each other. b. have a minimum level of kinetic energy. c. form an activated complex. d. all of the above ...
... c. neither a nor b b. chemical equilibrium. d. both a and b 8. According to collision theory, in order for a chemical reaction to occur, the reactant atoms must: a. make contact with each other. b. have a minimum level of kinetic energy. c. form an activated complex. d. all of the above ...
Thermodynamics Test Study Guide—AP _____ 1. The entropy
... 10. A student adds 200.0 grams of copper metal shot at 25.0oC to 200.0 mL of water at 80.0oC. The final temperature of the mixture is 75.3oC. Assuming that the specific heat of water is 1.00 cal/g-oC and that no heat is lost to or gained from the surroundings, what is the specific heat of copper, i ...
... 10. A student adds 200.0 grams of copper metal shot at 25.0oC to 200.0 mL of water at 80.0oC. The final temperature of the mixture is 75.3oC. Assuming that the specific heat of water is 1.00 cal/g-oC and that no heat is lost to or gained from the surroundings, what is the specific heat of copper, i ...
full text - pdf 452 kB
... This requires a knowledge of log K at the conditions (temperature, pressure, ionic strength) of the reaction. The log K values as well as the other thermodynamic quantities such as the AH,AS and ACp values associated with reactions in aqueous solutions can change in a dramatic fashion with temperatu ...
... This requires a knowledge of log K at the conditions (temperature, pressure, ionic strength) of the reaction. The log K values as well as the other thermodynamic quantities such as the AH,AS and ACp values associated with reactions in aqueous solutions can change in a dramatic fashion with temperatu ...
DOC
... conc of products are too large reaction shifts in reverse to reach equilibrium if Q < K conc of reactants are too large reaction shifts forward to reach equilibrium ...
... conc of products are too large reaction shifts in reverse to reach equilibrium if Q < K conc of reactants are too large reaction shifts forward to reach equilibrium ...
CERAMICS MATERIALS - Wits Structural Chemistry
... Synthetic reactions generally involves molecular rearrangements or substitution of one group or ligand by another. This requires small activation energies, low temperature (0 – 150 °C) and in solvents that aid diffusing reacting species. New materials can be obtained by two main methods; the breakin ...
... Synthetic reactions generally involves molecular rearrangements or substitution of one group or ligand by another. This requires small activation energies, low temperature (0 – 150 °C) and in solvents that aid diffusing reacting species. New materials can be obtained by two main methods; the breakin ...
Name ……………………………..………...… …….. Index No
... 7. In an experiment to determine the molar heat of neutralization of hydrochloric acid with sodium hydroride, students of Kassu Secondary school reacted 100cm3 of 1M hydrochloric acid with 50cm3 of 2M sodium hydroxide solution. They obtained the following results. Initial temperature of acid = 25.00 ...
... 7. In an experiment to determine the molar heat of neutralization of hydrochloric acid with sodium hydroride, students of Kassu Secondary school reacted 100cm3 of 1M hydrochloric acid with 50cm3 of 2M sodium hydroxide solution. They obtained the following results. Initial temperature of acid = 25.00 ...
Scientific Notation, Measurements, and
... Temperature is a measurement of how hot or cold something is. It is a calculation of the average kinetic energy of all the particles in the substance. Scientists theorize that at absolute zero, or –273.15oC, particle motion stops and that no more energy can be removed from the object. So this is the ...
... Temperature is a measurement of how hot or cold something is. It is a calculation of the average kinetic energy of all the particles in the substance. Scientists theorize that at absolute zero, or –273.15oC, particle motion stops and that no more energy can be removed from the object. So this is the ...
Honors Chemistry Final Review
... Numeral. The Roman Numeral indicates the charge of the metal ion, and while it is sometimes possible to determine this by sauce-apple-cross-criss, using algebra will always get you the correct answer. Also, remember that unless it ends in a polyatomic ion, the ending is always “ide”. When given the ...
... Numeral. The Roman Numeral indicates the charge of the metal ion, and while it is sometimes possible to determine this by sauce-apple-cross-criss, using algebra will always get you the correct answer. Also, remember that unless it ends in a polyatomic ion, the ending is always “ide”. When given the ...
Students know
... 8. Chemical reaction rates depend on factors that influence the frequency of collision of reactant molecules. As a basis for understanding this concept: a. Students know the rate of reaction is the decrease in concentration of reactants or the increase in concentration of products with time. b. Stud ...
... 8. Chemical reaction rates depend on factors that influence the frequency of collision of reactant molecules. As a basis for understanding this concept: a. Students know the rate of reaction is the decrease in concentration of reactants or the increase in concentration of products with time. b. Stud ...
Equilibrium (Sheet 1)
... Section III La Chatelier's principle states that if a stress such as a change in concentration, pressure or temperature is applied to a system in equilibrium, the equilibrium will shift in a way that tends to undo the effect of the stress. For example: H2O + CO H2 + CO2 + heat. If no stress is intro ...
... Section III La Chatelier's principle states that if a stress such as a change in concentration, pressure or temperature is applied to a system in equilibrium, the equilibrium will shift in a way that tends to undo the effect of the stress. For example: H2O + CO H2 + CO2 + heat. If no stress is intro ...