Energy Levels and Light Absorption
... • Consider an electron in an atom with quantum number n = n1. How many electrons in this atom can have the quantum number n1? n1 • Pauli exclusion says no two electrons can be in exactly the same state ...
... • Consider an electron in an atom with quantum number n = n1. How many electrons in this atom can have the quantum number n1? n1 • Pauli exclusion says no two electrons can be in exactly the same state ...
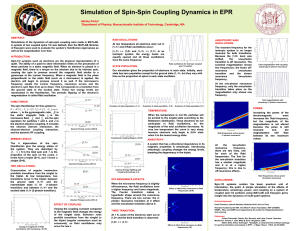
Simulation of Spin-Spin Coupling Dynamics in EPR
... causing the spin to precess around the magnetic field similar to a gyroscope at the Larmor frequency. When a magnetic field in the plane perpendicular to the static field (such as a microwave) is applied, applied, the electron will begin to precess around it as well. If the microwave microwave’’s fr ...
... causing the spin to precess around the magnetic field similar to a gyroscope at the Larmor frequency. When a magnetic field in the plane perpendicular to the static field (such as a microwave) is applied, applied, the electron will begin to precess around it as well. If the microwave microwave’’s fr ...
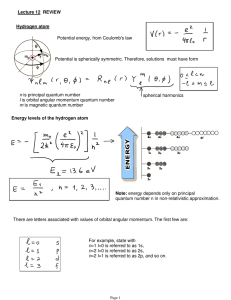
Lecture 12: Review.
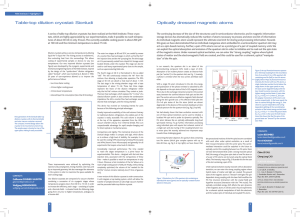
... moments with the electromagnetic fields of the electrons. The level splitting caused by this interaction is even smaller than the fine structure, so it is called hyperfine structure. We consider magnetic-dipole hyperfine interaction, i.e. the interaction of the nuclear magnetic moment with the magne ...
... moments with the electromagnetic fields of the electrons. The level splitting caused by this interaction is even smaller than the fine structure, so it is called hyperfine structure. We consider magnetic-dipole hyperfine interaction, i.e. the interaction of the nuclear magnetic moment with the magne ...
Slide1
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
... Spin is like angular momentum Recall m can have (2l+1) values between –l and l. For spin, since only 2 ...
T. Dammak - TU-MRS
... 2C6PbBr4, crystallises in a two-dimensional structure with a P21/a space group. In the inorganic semiconductor sub-lattice, the corner sharing CuCl6 octahedra form infinite 2D chains. The organic C4H16N3+ ions form the insulator barriers between the inorganic semiconductor layers. Such a packing lea ...
... 2C6PbBr4, crystallises in a two-dimensional structure with a P21/a space group. In the inorganic semiconductor sub-lattice, the corner sharing CuCl6 octahedra form infinite 2D chains. The organic C4H16N3+ ions form the insulator barriers between the inorganic semiconductor layers. Such a packing lea ...
Alkali Elements Alkali Elements: Excited States
... Most of the energetics of these atoms is well described by the Hartree model; however, in detail (e.g. in high-resolution spectroscopy), spin-orbit coupling and the residual coulomb interaction are important. Residual Coulomb Interaction: The Coulomb interaction that is not captured by the effective ...
... Most of the energetics of these atoms is well described by the Hartree model; however, in detail (e.g. in high-resolution spectroscopy), spin-orbit coupling and the residual coulomb interaction are important. Residual Coulomb Interaction: The Coulomb interaction that is not captured by the effective ...
Lecture 4
... Note on spectroscopic notations (they are actually used). There are letters associated with values of orbital angular momentum. The first few are: For example, state with n=1 l=0 is referred to as 1s, n=2 l=0 is referred to as 2s, n=2 l=1 is referred to as 2p, and so on. While the energies are the s ...
... Note on spectroscopic notations (they are actually used). There are letters associated with values of orbital angular momentum. The first few are: For example, state with n=1 l=0 is referred to as 1s, n=2 l=0 is referred to as 2s, n=2 l=1 is referred to as 2p, and so on. While the energies are the s ...
Nuclear Magnetic Resonance Spectroscopy
... electromagnetic radiation in the radio frequency region of roughly 4 to 900MHz. In contrast to UV, IR and visible absorption, nuclei of atoms rather than outer electrons are involved in the process. In order to cause nuclei to develop the energy states required for absorption to occur, it is necessa ...
... electromagnetic radiation in the radio frequency region of roughly 4 to 900MHz. In contrast to UV, IR and visible absorption, nuclei of atoms rather than outer electrons are involved in the process. In order to cause nuclei to develop the energy states required for absorption to occur, it is necessa ...
Chapter 7 Handout 1 Atomic Orbitals Quantum Numbers: Principal
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
ESR Theory - Personal WWW Pages
... As seen previously, the electron spin energy levels are split by the magnetic field. In addition there is further splitting due to the four possible values of m I. Since the coupling is to a nucleus of spin I = 3/2, there should be 2n(I + 1) lines (ie 2.1.(3/2 +1) = 4 lines). The four transitions ar ...
... As seen previously, the electron spin energy levels are split by the magnetic field. In addition there is further splitting due to the four possible values of m I. Since the coupling is to a nucleus of spin I = 3/2, there should be 2n(I + 1) lines (ie 2.1.(3/2 +1) = 4 lines). The four transitions ar ...
Nitrogen-vacancy center

The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual N-V center, especially those in the negative charge state (N-V−). Electron spins at N-V centers, localized at atomic scales, can be manipulated at room temperature by applying a magnetic field, electric field, microwave radiation or light, or a combination, resulting in sharp resonances in the intensity and wavelength of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin-orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual N-V center can be viewed as a basic unit of a quantum computer, and it has potential applications in novel, more efficient fields of electronics and computational science including quantum cryptography and spintronics.