Nugget
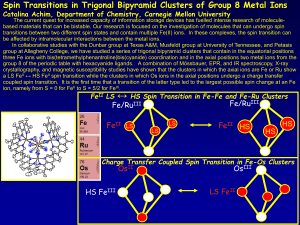
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
1 - TAMU Chemistry
... c) According to your assignment for the divalent carbon ligand, give the oxidation state of the metal and the d-electron count in each case. d) Which divalent carbon ligand is most likely to react with PhLi? What is the product? e) Which divalent carbon ligand is most likely to have resulted from an ...
... c) According to your assignment for the divalent carbon ligand, give the oxidation state of the metal and the d-electron count in each case. d) Which divalent carbon ligand is most likely to react with PhLi? What is the product? e) Which divalent carbon ligand is most likely to have resulted from an ...
Atomic Spectra - Flinn Scientific
... The phenomenon of atomic spectra has been known since the mid-1800s. Their cause, however, remained unexplained until the structure of the atom and, in particular, its electronic structure, was solved. Rutherford’s discovery of the nucleus of the atom in 1911 answered many questions concerning the s ...
... The phenomenon of atomic spectra has been known since the mid-1800s. Their cause, however, remained unexplained until the structure of the atom and, in particular, its electronic structure, was solved. Rutherford’s discovery of the nucleus of the atom in 1911 answered many questions concerning the s ...
Atomic spectra
... transitions that occur most often, that is, they have the highest probability of occurring. A spectral line is very weak when the transition has a low probability of occurring. When atoms are in an external magnetic field, the individual energy levels may be split into a number of separate energy le ...
... transitions that occur most often, that is, they have the highest probability of occurring. A spectral line is very weak when the transition has a low probability of occurring. When atoms are in an external magnetic field, the individual energy levels may be split into a number of separate energy le ...
IB Physics Quantum Physics Schrodinger, Uncertainty Principle, and
... This question is about atomic spectra and energy levels. Diagram 1 below shows part of the emission line spectrum of atomic hydrogen. The wavelengths of the principal lines in the visible region of the spectrum are shown. Diagram 2 shows some of the principal energy levels of atomic hydrogen. ...
... This question is about atomic spectra and energy levels. Diagram 1 below shows part of the emission line spectrum of atomic hydrogen. The wavelengths of the principal lines in the visible region of the spectrum are shown. Diagram 2 shows some of the principal energy levels of atomic hydrogen. ...
Nuclear Structure - UNLV Radiochemistry
... • Nuclei with non-zero spin have magnetic moments • Protons and neutrons have magnetic moments for neutrons positive charge in center, negative charge on periphery Experimental Evidence of Spin and Magnetic Moments • Hyperfine structure in atomic spectra nuclear and electronic magnetic interacti ...
... • Nuclei with non-zero spin have magnetic moments • Protons and neutrons have magnetic moments for neutrons positive charge in center, negative charge on periphery Experimental Evidence of Spin and Magnetic Moments • Hyperfine structure in atomic spectra nuclear and electronic magnetic interacti ...
Physics Review
... B. melting point is T2 C. specific heat is greater for the liquid phase than for the solid phase D. heat of fusion and heat of vaporization are equal E. specific heat of the solid increases linearly with ...
... B. melting point is T2 C. specific heat is greater for the liquid phase than for the solid phase D. heat of fusion and heat of vaporization are equal E. specific heat of the solid increases linearly with ...
Magnetic-Field Induced Enhancement in the Fluorescence Yield Spectrum
... FY spectrum is due to mixing with states that have larger FBR than the states reached in the field-free case, in analogy to the electric-field effects [9]. However, whereas the eigenenergies fan symmetrically out from each n manifold with increasing electric field, the energies for the diamagnetic e ...
... FY spectrum is due to mixing with states that have larger FBR than the states reached in the field-free case, in analogy to the electric-field effects [9]. However, whereas the eigenenergies fan symmetrically out from each n manifold with increasing electric field, the energies for the diamagnetic e ...
Nuclear Physics
... absorb photons that not only ionize the metal, but give the electron enough kinetic energy to escape from the atom and travel ...
... absorb photons that not only ionize the metal, but give the electron enough kinetic energy to escape from the atom and travel ...
Chemistry 882: Spectroscopy and Kinetics
... Mechanisms, Catalysis and Enzymes, and Atmospheric Kinetics. It will be given during the Final Exam time, Monday, May 3, 12:45 p.m. – 2:45 p.m. A review sheet will be prepared for each exam. In addition, formulas and constants will be provided on the exam, unless the exam question explicitly asks yo ...
... Mechanisms, Catalysis and Enzymes, and Atmospheric Kinetics. It will be given during the Final Exam time, Monday, May 3, 12:45 p.m. – 2:45 p.m. A review sheet will be prepared for each exam. In addition, formulas and constants will be provided on the exam, unless the exam question explicitly asks yo ...
Photoelectric Effect www.AssignmentPoint.com The photoelectric
... function of a sample can be determined by bombarding it with a monochromatic X-ray source or UV source, and measuring the kinetic energy distribution of the electrons emitted. ...
... function of a sample can be determined by bombarding it with a monochromatic X-ray source or UV source, and measuring the kinetic energy distribution of the electrons emitted. ...
Electronic Structure - Chemistry Teaching Resources
... The Pauli Exclusion Principle states that the maximum number of electrons in any atomic orbital is two…….. and ….. if there are two electrons in an orbital they must have opposite spins (rather than parallel spins). Aug 2016 ...
... The Pauli Exclusion Principle states that the maximum number of electrons in any atomic orbital is two…….. and ….. if there are two electrons in an orbital they must have opposite spins (rather than parallel spins). Aug 2016 ...
TR-12
... Deuteron’s nuclear spin quantum number is 1. This indicates the neutron and proton spins are aligned parallel to each other. The nuclear magnetic moment of a deuteron is 0.86μN ≈ the sum of the free proton and neutron 2.79μN − 1.91μN = 0.88μN. ...
... Deuteron’s nuclear spin quantum number is 1. This indicates the neutron and proton spins are aligned parallel to each other. The nuclear magnetic moment of a deuteron is 0.86μN ≈ the sum of the free proton and neutron 2.79μN − 1.91μN = 0.88μN. ...
Document
... the transition from n to n+1 corresponds to the energy of the absorbed light quantum • The difference between two adjacent energy levels gets smaller with increasing n until dissociation of the molecule occurs (Dissociation energy ED ) Note: Weaker transitions called “overtones” are sometimes observ ...
... the transition from n to n+1 corresponds to the energy of the absorbed light quantum • The difference between two adjacent energy levels gets smaller with increasing n until dissociation of the molecule occurs (Dissociation energy ED ) Note: Weaker transitions called “overtones” are sometimes observ ...
Laboratory Pb Name: Date: ______ (1) Measure the mass of a
... (5) Table S in the Chemistry Reference Tables lists the accepted value for the density of lead. Calculate your percent error. Show all work. ...
... (5) Table S in the Chemistry Reference Tables lists the accepted value for the density of lead. Calculate your percent error. Show all work. ...
Organometallic Chemistry at the Magnesium− Tris (8
... O(1s) are not so easily interpreted in terms of a model in which the quinolinate ligands of Alq3 undergo simple reduction: These calculations show that, even though the LUMO is maximized on the pyridyl ring, some increase in negative charge also accrues to the phenolic ring of the quinolinate ligand ...
... O(1s) are not so easily interpreted in terms of a model in which the quinolinate ligands of Alq3 undergo simple reduction: These calculations show that, even though the LUMO is maximized on the pyridyl ring, some increase in negative charge also accrues to the phenolic ring of the quinolinate ligand ...
General Physics I - University of Rochester
... • Potential energy of the magnetic dipole in magnetic field splits into several levels ...
... • Potential energy of the magnetic dipole in magnetic field splits into several levels ...
Pre-Test: 2nd semester Final Exam Review File
... c. Light energy is converted to mechanical energy d. Nuclear energy is converted into chemical energy 37. What are the seven forms of energy? a. Chemical, mechanical, heat, sound, light, motion, and nuclear b. Mechanical, heat, light, speed, velocity, potential and wind c. Sound, light, heat, grassh ...
... c. Light energy is converted to mechanical energy d. Nuclear energy is converted into chemical energy 37. What are the seven forms of energy? a. Chemical, mechanical, heat, sound, light, motion, and nuclear b. Mechanical, heat, light, speed, velocity, potential and wind c. Sound, light, heat, grassh ...
presentation - WordPress.com
... an extra electron is added to an atom. For the bond formation electron gain enthalpy of an element should be high. ...
... an extra electron is added to an atom. For the bond formation electron gain enthalpy of an element should be high. ...
File
... 1. The spectrum of a solid sample is determined with an alkali halide pellet. About 1-3 mg of substance and 100-200 mg of alkali halide are ground together, dried to remove moisture and pressed at room temperature under high pressure into a small disc. KBr does not absorb IR radiation in the region ...
... 1. The spectrum of a solid sample is determined with an alkali halide pellet. About 1-3 mg of substance and 100-200 mg of alkali halide are ground together, dried to remove moisture and pressed at room temperature under high pressure into a small disc. KBr does not absorb IR radiation in the region ...
Study guide for Tools of Astronomy
... (radio, microwave, infrared, ultraviolet, x-ray, and gamma ray) Know the arrangement of the EM spectrum, by wavelength Know what the Doppler effect is and how it is used by astronomers (blue shift, red shift) and what the significance is of being able to detect those shifts in light. Know the name o ...
... (radio, microwave, infrared, ultraviolet, x-ray, and gamma ray) Know the arrangement of the EM spectrum, by wavelength Know what the Doppler effect is and how it is used by astronomers (blue shift, red shift) and what the significance is of being able to detect those shifts in light. Know the name o ...
LIGHT APLIFICATION by STIMULATED EMISSION of RADITIONS
... of energy hƒ= E1- E0 strikes the atom in excited state , it comes to ground state. The photon emitted goes parallel to incident photon & both of these photons are in phase. For laser to have Stimulated Emission, the following two conditions must be satisfied 1)The higher energy state should have lon ...
... of energy hƒ= E1- E0 strikes the atom in excited state , it comes to ground state. The photon emitted goes parallel to incident photon & both of these photons are in phase. For laser to have Stimulated Emission, the following two conditions must be satisfied 1)The higher energy state should have lon ...
Physical Science
... temperature of the mixture every 5 seconds. They continue these observations until 10 seconds after they see the mixture stop bubbling. Their data are shown in the table below. The data show that the mass of the cup and its contents decreases while the mixture is bubbling. Explain why the mass of th ...
... temperature of the mixture every 5 seconds. They continue these observations until 10 seconds after they see the mixture stop bubbling. Their data are shown in the table below. The data show that the mass of the cup and its contents decreases while the mixture is bubbling. Explain why the mass of th ...
Absorption & Emission
... a is the chromophore area in cm2 The transition probability depends on a number of factors including where the transition is an “allowed” transition or a “forbidden” transition ...
... a is the chromophore area in cm2 The transition probability depends on a number of factors including where the transition is an “allowed” transition or a “forbidden” transition ...
Journal of Babylon University/Pure and Applied Sciences/ No.(6
... of nuclei can described in terms of a monopole bosons with angular momentum and parity J P 0 ,called s and a quadrupole boson with J 2 called d (Iachello et al,1987). There are two basic concepts on which the IBM is based. One is that low-lying collective states in even-even nuclei can be descr ...
... of nuclei can described in terms of a monopole bosons with angular momentum and parity J P 0 ,called s and a quadrupole boson with J 2 called d (Iachello et al,1987). There are two basic concepts on which the IBM is based. One is that low-lying collective states in even-even nuclei can be descr ...
Mössbauer spectroscopy

Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer in 1957, consists in the recoil-free, resonant absorption and emission of gamma rays in solids.Like NMR spectroscopy, Mössbauer spectroscopy probes tiny changes in the energy levels of an atomic nucleus in response to its environment. Typically, three types of nuclear interactions may be observed: an isomeric shift, also known as a chemical shift; quadrupole splitting; and magnetic or hyperfine splitting, also known as the Zeeman effect. Due to the high energy and extremely narrow line widths of gamma rays, Mössbauer spectroscopy is a very sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting change in just a few parts per 1011.