Quantum Numbers
... • Excited state: Higher potential energy than ground state. • Photon: A particle of electromagnetic radiation having zero mass and carrying a quantum of energy (i.e., packet of light) • Only certain wavelengths of light are emitted by hydrogen atoms when electric current is passed through—Why? Mulli ...
... • Excited state: Higher potential energy than ground state. • Photon: A particle of electromagnetic radiation having zero mass and carrying a quantum of energy (i.e., packet of light) • Only certain wavelengths of light are emitted by hydrogen atoms when electric current is passed through—Why? Mulli ...
Magnetic Fields 2
... terminal of the power supply. Connect the other end of the wire to the negative terminal. Adjust the current to about 2 amperes. Disconnect the wire from the positive terminal. Assuming that positive current comes out of the positive terminal, sketch which direction positive current flows through th ...
... terminal of the power supply. Connect the other end of the wire to the negative terminal. Adjust the current to about 2 amperes. Disconnect the wire from the positive terminal. Assuming that positive current comes out of the positive terminal, sketch which direction positive current flows through th ...
Modern Physics 342
... Two opposite dipoles in the same non-uniform electric field are affected by opposite net forces that lead to displacing each dipole up and down according to their respective alignments. ...
... Two opposite dipoles in the same non-uniform electric field are affected by opposite net forces that lead to displacing each dipole up and down according to their respective alignments. ...
MRI
... neutrons all posses spin, either + or – ½. Because of the positive and negative factors, spins can pair up and cancel each other. Unpaired, nuclear spins are utilized in NMR. However, NMR can only be performed on isotopes whose natural abundance is high enough for detection. Within a magnetic field, ...
... neutrons all posses spin, either + or – ½. Because of the positive and negative factors, spins can pair up and cancel each other. Unpaired, nuclear spins are utilized in NMR. However, NMR can only be performed on isotopes whose natural abundance is high enough for detection. Within a magnetic field, ...
Name
... This model does not specify an exact path an electron takes around the nucleus, but gives the probability of finding an electron within a certain volume of space around the nucleus. This volume of space is described as an electron cloud, which has no boundary. The electron cloud is denser where the ...
... This model does not specify an exact path an electron takes around the nucleus, but gives the probability of finding an electron within a certain volume of space around the nucleus. This volume of space is described as an electron cloud, which has no boundary. The electron cloud is denser where the ...
Electron Structure of Atoms Notes
... electrons in an atom. There are 4 quantum numbers Principal quantum number (n) Azimuthual quantum number (angular momentum) (ℓ) Magnetic quantum number (mℓ) Electron spin quantum number (ms) ...
... electrons in an atom. There are 4 quantum numbers Principal quantum number (n) Azimuthual quantum number (angular momentum) (ℓ) Magnetic quantum number (mℓ) Electron spin quantum number (ms) ...
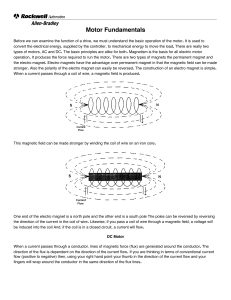
Inductor Basics notes
... Inductance An inductor is a series of turns of wire around a core material. The core can be air, soft iron in solid of powdered form, graphite or alloys of different metals chosen for their magnetic properties. The inductance of a coil (inductor) is dependant on: 1. Number of turns 2. Area (How clo ...
... Inductance An inductor is a series of turns of wire around a core material. The core can be air, soft iron in solid of powdered form, graphite or alloys of different metals chosen for their magnetic properties. The inductance of a coil (inductor) is dependant on: 1. Number of turns 2. Area (How clo ...
ELE 100 Introduction to Engineering
... We will use the power supply as a current supply. Set the voltage of the power supply to 1 volt. Turn the current limit down to as low as it goes. Place the wooden wire support so the wire is horizontal. Connect the end of the wire with a dot on the post to the positive terminal of the power supply. ...
... We will use the power supply as a current supply. Set the voltage of the power supply to 1 volt. Turn the current limit down to as low as it goes. Place the wooden wire support so the wire is horizontal. Connect the end of the wire with a dot on the post to the positive terminal of the power supply. ...
Spin-Orbit Suppression of Cold Inelastic Collisions of Aluminum and Helium Please share
... collisions of atoms in 2 P1=2 states (e.g., Al, Ga, In, and Tl, and metastable halogens) with He was supported by experiments done in tandem that observed large suppression in both Ga and In [13], but the very low rate constant for mJ -changing collisions (a critical process for magnetic trapping) w ...
... collisions of atoms in 2 P1=2 states (e.g., Al, Ga, In, and Tl, and metastable halogens) with He was supported by experiments done in tandem that observed large suppression in both Ga and In [13], but the very low rate constant for mJ -changing collisions (a critical process for magnetic trapping) w ...
Document
... Similar terms are added for torsion angles from J-coupling and for orientations of bonds from RDCs ...
... Similar terms are added for torsion angles from J-coupling and for orientations of bonds from RDCs ...
Name
... The aufbau principle says that electrons occupy the orbitals of lowest energy first. According to the Pauli exclusion principle, each orbital can contain at most two electrons. The two electrons must have opposite spin. Hund’s rule states that single electrons occupy orbitals in a specific sublevel ...
... The aufbau principle says that electrons occupy the orbitals of lowest energy first. According to the Pauli exclusion principle, each orbital can contain at most two electrons. The two electrons must have opposite spin. Hund’s rule states that single electrons occupy orbitals in a specific sublevel ...
Document
... An electron’s position in an atom or ion can be described by determining its electron configuration and orbital diagram. These representations of an atom or ion can explain physical and chemical properties of the substance, including magnetic attraction. Bohr Atom Niels Bohr’s “Planetary Model”- in ...
... An electron’s position in an atom or ion can be described by determining its electron configuration and orbital diagram. These representations of an atom or ion can explain physical and chemical properties of the substance, including magnetic attraction. Bohr Atom Niels Bohr’s “Planetary Model”- in ...
Atomic structure review
... Atomic structure review - H People Thompson – discovered electrons Rutherford – discovered the nucleus – small dense positive nucleus, volume empty space Bohr – electrons have quantized (specific) energy, shell model Heisenberg – due to wave nature of electrons you can’t know the position and moment ...
... Atomic structure review - H People Thompson – discovered electrons Rutherford – discovered the nucleus – small dense positive nucleus, volume empty space Bohr – electrons have quantized (specific) energy, shell model Heisenberg – due to wave nature of electrons you can’t know the position and moment ...
g-2 , muon edm and deuteron edm at a high intensity storage ring
... component of E field, must be kept at the 10-6 level. This has already been achieved (for B field) in g-2 BNL experiment. ...
... component of E field, must be kept at the 10-6 level. This has already been achieved (for B field) in g-2 BNL experiment. ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.