BCK0103-15 Quantum physics (3-0-4) - nuvem
... BCK0103-15 Quantum physics (3-0-4) General goals: The main goal of this course is to present to the student the main concepts of the quantum theory, with the perspective of comprehending the basic phenomena which originate at the atomic scale, their effects and technological applications. ...
... BCK0103-15 Quantum physics (3-0-4) General goals: The main goal of this course is to present to the student the main concepts of the quantum theory, with the perspective of comprehending the basic phenomena which originate at the atomic scale, their effects and technological applications. ...
Quantum mechanics is the theory that we use to describe the
... Quantum mechanics is also an inherently probabilistic theory, in that there exists uncertainty at the most fundamental level when we try to measure any value, or observable, of a system. This is unlike classical and relativistic theories, where everything exists with a precise and definite values, a ...
... Quantum mechanics is also an inherently probabilistic theory, in that there exists uncertainty at the most fundamental level when we try to measure any value, or observable, of a system. This is unlike classical and relativistic theories, where everything exists with a precise and definite values, a ...
Chemistry Science Notebook
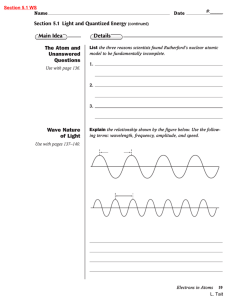
... List the three reasons scientists found Rutherford’s nuclear atomic model to be fundamentally incomplete. ...
... List the three reasons scientists found Rutherford’s nuclear atomic model to be fundamentally incomplete. ...
... Eq. (2) one finds that A commutes with H and, therefore, if A does not depend on the time, then A is conserved. It should be pointed out that Refs. 8 and 9 also consider the Galilean transformations, which are related to a constant of motion that depends explicitly on the time (see Sec. 3.1, below). ...
cm16_1
... • The piano has two or three strings for each note • When a key is pressed, the hammer strikes these strings simultaneously ...
... • The piano has two or three strings for each note • When a key is pressed, the hammer strikes these strings simultaneously ...
Aug 29 - BYU Physics and Astronomy
... Introduction to Quantum mechanics Essential ideas 1) Uncertainty principle: Conjugates quantities of a particle (ex: position & momentum) can not be known simultaneously within a certain accuracy limit 2) Quantization: The measurement of a physical quantity in a confined system results in quanta (t ...
... Introduction to Quantum mechanics Essential ideas 1) Uncertainty principle: Conjugates quantities of a particle (ex: position & momentum) can not be known simultaneously within a certain accuracy limit 2) Quantization: The measurement of a physical quantity in a confined system results in quanta (t ...
Slide 1
... oAn atom with its e- in the lowest possible energy levels is said to be in its “ground state” oWhen an e- occupies an orbit greater than the lowest possible energy level it is said to be in an “excited state” oΔE=-Rhc(1/nf2 - 1/ni2) Rhc=1312 kJ/mol Wave/particle duality oTaken from idea that light ...
... oAn atom with its e- in the lowest possible energy levels is said to be in its “ground state” oWhen an e- occupies an orbit greater than the lowest possible energy level it is said to be in an “excited state” oΔE=-Rhc(1/nf2 - 1/ni2) Rhc=1312 kJ/mol Wave/particle duality oTaken from idea that light ...
Sec 4-1 Chapter 4 Notes
... In 1926, Erwin Schrodinger developed an equation that treated e- as waves. Together, the Heisenberg uncertainty principle and the Schrodinger equation laid the foundation for the Quantum theory. Heisenberg uncertainty principle gives us a probability of where to find the e-. The e- does not travel ...
... In 1926, Erwin Schrodinger developed an equation that treated e- as waves. Together, the Heisenberg uncertainty principle and the Schrodinger equation laid the foundation for the Quantum theory. Heisenberg uncertainty principle gives us a probability of where to find the e-. The e- does not travel ...
science 1 small-group tutorial scheme
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
... List all the subshells and orbitals for which the principal quantum number, n, has the values 2 and 3 in order of increasing energy, according to the quantum mechanical rules. Use clearly-labelled diagrams to illustrate the shapes and orientations of the orbitals for which the angular momentum (subs ...
polarization of the allotropic hollow foms of carbon and its use in
... The analytical model of polarizing resonant interactions hollow forms of carbon with the quantum charged particles with total energy E > 0 is offered. The problem is shown to classical quantum-mechanical effect: «a particle in a box» (a Q-particle) in which power conditions are defined by the sizes ...
... The analytical model of polarizing resonant interactions hollow forms of carbon with the quantum charged particles with total energy E > 0 is offered. The problem is shown to classical quantum-mechanical effect: «a particle in a box» (a Q-particle) in which power conditions are defined by the sizes ...
Quantum Mechanics and Applications
... 2.1 Quantum theory The quantum theory was proposed by Max Planck in 1900. This theory deals with the body of science principles that explains the behavior of matter and its interaction with energy on the scale of atoms and subatomic particles, mainly with the photons. This theory overcome all the fa ...
... 2.1 Quantum theory The quantum theory was proposed by Max Planck in 1900. This theory deals with the body of science principles that explains the behavior of matter and its interaction with energy on the scale of atoms and subatomic particles, mainly with the photons. This theory overcome all the fa ...
Welcome to Physics 112N
... The Ultraviolet Catastrophe Classical physics can describe the shape of the blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
... The Ultraviolet Catastrophe Classical physics can describe the shape of the blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
Lecture 26 - Purdue Physics
... Atomic Quantum Numbers • Sommerfeld extended the Bohr model to account for quantized angular momentum • A new quantum number, ℓ, known as the orbital quantum number, identifies the orbital angular momentum of a state. ...
... Atomic Quantum Numbers • Sommerfeld extended the Bohr model to account for quantized angular momentum • A new quantum number, ℓ, known as the orbital quantum number, identifies the orbital angular momentum of a state. ...
The Learnability of Quantum States
... Quantum Computing and the Interpretation of Quantum Mechanics? David Deutsch’s argument for Many Worlds: “To those who still cling to a single-universe worldview, I issue this challenge: explain how Shor's algorithm works … When Shor's algorithm has factorized a number, using 10⁵⁰⁰ or so times the ...
... Quantum Computing and the Interpretation of Quantum Mechanics? David Deutsch’s argument for Many Worlds: “To those who still cling to a single-universe worldview, I issue this challenge: explain how Shor's algorithm works … When Shor's algorithm has factorized a number, using 10⁵⁰⁰ or so times the ...
Spectroscopy - Birmingham City Schools
... n = principle quantum number (energy level) l = orbital quantum number (shape of orbit) ml = magnetic quantum number ...
... n = principle quantum number (energy level) l = orbital quantum number (shape of orbit) ml = magnetic quantum number ...
A Note on the Quantum Mechanical Time Reversal - Philsci
... these must be adopted. In *(QM), we take the wave: * to represent a particle with the same classical properties as in QM – this is the basic isomorphism. Alternatively, in T(QM), T represents a particle with the time-reversed classical properties represented by in QM. Now, for instance, consid ...
... these must be adopted. In *(QM), we take the wave: * to represent a particle with the same classical properties as in QM – this is the basic isomorphism. Alternatively, in T(QM), T represents a particle with the time-reversed classical properties represented by in QM. Now, for instance, consid ...