
6. Macroscopic equilibrium states and state variables (Hiroshi
... The operational definition of temperature In Sec.4.1, we have adopted the following operational definition for temperature: temperature is a macroscopic quantity that we measure using a thermometer. Below we will explain what we mean by this definition. As the notion of temperature is derived from a ...
... The operational definition of temperature In Sec.4.1, we have adopted the following operational definition for temperature: temperature is a macroscopic quantity that we measure using a thermometer. Below we will explain what we mean by this definition. As the notion of temperature is derived from a ...
Fractional Distillation
... packing. It is important for fairly small samples that the hold-up be relatively small. The number of theoretical plates is a measure of how many times the liquid undergoes the enrichment process. Typical laboratory scale fractionating columns will have 6-8 theoretical plates. It is important to kee ...
... packing. It is important for fairly small samples that the hold-up be relatively small. The number of theoretical plates is a measure of how many times the liquid undergoes the enrichment process. Typical laboratory scale fractionating columns will have 6-8 theoretical plates. It is important to kee ...
Analysis of process-induced residual stresses in tape placement
... incompatible. Although elastic properties of fibers are almost unaffected by temperature, influence of temperature on the properties of the polymer matrix is drastic. Also high processing temperatures typical for high performance thermoplastic composites exacerbate both the effect of temperature gra ...
... incompatible. Although elastic properties of fibers are almost unaffected by temperature, influence of temperature on the properties of the polymer matrix is drastic. Also high processing temperatures typical for high performance thermoplastic composites exacerbate both the effect of temperature gra ...
basic thermodynamics
... Gas is cooled from state 1 to state 2 (Fig). For reversible heat transfer, the working fluid in the heat engine would have been heated along 2-1, so that at any instant, the temperature difference between gas and the working fluid is zero. Then 1-b would have been the expansion of the working fluid ...
... Gas is cooled from state 1 to state 2 (Fig). For reversible heat transfer, the working fluid in the heat engine would have been heated along 2-1, so that at any instant, the temperature difference between gas and the working fluid is zero. Then 1-b would have been the expansion of the working fluid ...
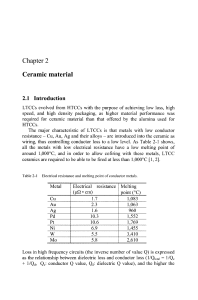
Chapter 2 Ceramic material
... 738"C), LiF, B203,Bi203,Pb5Ge2.4Si0.6011 (melting point: 750°C), Pb2Si04 (melting point: 750°C), Li2Bi205(melting point: 700°C) and so on are known as sintering additives that are added to ceramics at less than 10 wt% [7, 8,9], but they are not typical of LTCCs. This chapter focuses on the composite ...
... 738"C), LiF, B203,Bi203,Pb5Ge2.4Si0.6011 (melting point: 750°C), Pb2Si04 (melting point: 750°C), Li2Bi205(melting point: 700°C) and so on are known as sintering additives that are added to ceramics at less than 10 wt% [7, 8,9], but they are not typical of LTCCs. This chapter focuses on the composite ...
2.26 MB - KFUPM Resources v3
... Compressed liquid means that it is not about to vaporize. The system is heated and the piston is allowed to float and thus the pressure will be constant. T and v will increase until the system reaches 100 C at which any addition of heat will cause some of the liquid to vaporize The temperature at wh ...
... Compressed liquid means that it is not about to vaporize. The system is heated and the piston is allowed to float and thus the pressure will be constant. T and v will increase until the system reaches 100 C at which any addition of heat will cause some of the liquid to vaporize The temperature at wh ...
notation for states and processes, significance of the word standard
... •the symbol for a crystalline solid is cr; where polymorphism occurs, it may be necessary to augment the symbol cr with a descriptor for the crystal modification under discussion; the preferred descriptors are Roman numerals, with textual definition of the crystallographic significance of the numera ...
... •the symbol for a crystalline solid is cr; where polymorphism occurs, it may be necessary to augment the symbol cr with a descriptor for the crystal modification under discussion; the preferred descriptors are Roman numerals, with textual definition of the crystallographic significance of the numera ...
6 The ocean lithosphere
... Equation 6.3, derived entirely from theoretical considerations, is in excellent agreement with observed bathymetry of ocean lithosphere younger than about 80 Ma. Indeed, this remarkable agreement between observations and the age-heatflow-bathymetry relationships predicted by Equations 6.1 - 6.3 prov ...
... Equation 6.3, derived entirely from theoretical considerations, is in excellent agreement with observed bathymetry of ocean lithosphere younger than about 80 Ma. Indeed, this remarkable agreement between observations and the age-heatflow-bathymetry relationships predicted by Equations 6.1 - 6.3 prov ...
thermodynamics type 1
... Chemical reactions are generally carried out at constant pressure (atmospheric pressure) so it has been found useful to define a new state function Enthalpy (H) as : H = E + PV (By definition) or ∆H = ∆E + P ∆V + V∆P or ∆H = ∆E + P ∆V (at constant pressure) combining with first law. Equation (1) bec ...
... Chemical reactions are generally carried out at constant pressure (atmospheric pressure) so it has been found useful to define a new state function Enthalpy (H) as : H = E + PV (By definition) or ∆H = ∆E + P ∆V + V∆P or ∆H = ∆E + P ∆V (at constant pressure) combining with first law. Equation (1) bec ...
Sample pages 2 PDF
... More complex to describe is the non-homogeneous system, in which some of the variables, for example, the temperature or the pressure, vary from point to point. To study them, one must divide the system into parts that are small enough to be able to be considered homogeneous. We shall not deal with a ...
... More complex to describe is the non-homogeneous system, in which some of the variables, for example, the temperature or the pressure, vary from point to point. To study them, one must divide the system into parts that are small enough to be able to be considered homogeneous. We shall not deal with a ...
[PDF]
... and the microwave oven cavity and waveguide were meshed for the microwave field. Power densities obtained on the structured FDTD mesh were mapped onto the unstructured finite volume method mesh for each time-step/turntable position. On heating for each specified time-step the temperature field was m ...
... and the microwave oven cavity and waveguide were meshed for the microwave field. Power densities obtained on the structured FDTD mesh were mapped onto the unstructured finite volume method mesh for each time-step/turntable position. On heating for each specified time-step the temperature field was m ...
Chp-1. Pure substances
... phases i.e. steam and ice, and also it is almost freely available as gift of nature. Pure substance refers to the substance with chemical homogenecity and constant chemical composition. Water is a pure substance it meets both the above requirements. Any substance, if it undergoes a chemical reaction ...
... phases i.e. steam and ice, and also it is almost freely available as gift of nature. Pure substance refers to the substance with chemical homogenecity and constant chemical composition. Water is a pure substance it meets both the above requirements. Any substance, if it undergoes a chemical reaction ...
- University of Surrey
... factors including the applied electric field and temperature. [14]–[17] An electrocaloric effect caused by ferroelectric switching therefore would create a feedback where the temperature change brought about by the electrocaloric effect affects the rate of ferroelectric switching and vice versa. Sim ...
... factors including the applied electric field and temperature. [14]–[17] An electrocaloric effect caused by ferroelectric switching therefore would create a feedback where the temperature change brought about by the electrocaloric effect affects the rate of ferroelectric switching and vice versa. Sim ...
- WRAP: Warwick Research Archive Portal
... limitations from the complexity and suspect relation to physical quantities may be overcome using the diffusive representation (state-space model) described in [12]. Related to thermal and electrical model complexity are the amount of relevant physics captured and accurately described by various mod ...
... limitations from the complexity and suspect relation to physical quantities may be overcome using the diffusive representation (state-space model) described in [12]. Related to thermal and electrical model complexity are the amount of relevant physics captured and accurately described by various mod ...
Anomalous thermodynamic properties in ferropericlase throughout
... transport in the mantle. Therefore, understanding the thermodynamics properties of these materials at lower mantle conditions is important to build more realistic Earth’s models.15 Fp is a solid solution of rocksalt-type MgO and FeO 共Mg1−xFexO兲 with FeO being a minor component 共x ⬃ 0.18 in the mantl ...
... transport in the mantle. Therefore, understanding the thermodynamics properties of these materials at lower mantle conditions is important to build more realistic Earth’s models.15 Fp is a solid solution of rocksalt-type MgO and FeO 共Mg1−xFexO兲 with FeO being a minor component 共x ⬃ 0.18 in the mantl ...
First Law of Thermodynamics – Basic Concepts
... parameters. It also stands to reason that a change of system from the initial state to the final state (2nd state) will be accompanied by change in the state variables. It is not necessary to state all the properties (state variables) to define a system completely. For a pure gas, the composition is ...
... parameters. It also stands to reason that a change of system from the initial state to the final state (2nd state) will be accompanied by change in the state variables. It is not necessary to state all the properties (state variables) to define a system completely. For a pure gas, the composition is ...
18 The First Law of Thermodynamics
... Temperature and its measurement are central to understanding the behavior of macroscopic systems that are heated and cooled. Although we have a natural ability to sense hot and cold, we can only use our sense of touch to tell whether an object is hot or cold over a relatively narrow range of temper ...
... Temperature and its measurement are central to understanding the behavior of macroscopic systems that are heated and cooled. Although we have a natural ability to sense hot and cold, we can only use our sense of touch to tell whether an object is hot or cold over a relatively narrow range of temper ...














![[PDF]](http://s1.studyres.com/store/data/008813345_1-ff859aabf04e6969803c0b58ad89f914-300x300.png)







