thermodynamics properties of pure substances
... Some tables may not always give the internal energy. When it is not listed, the internal energy is calculated from the definition of the enthalpy as ...
... Some tables may not always give the internal energy. When it is not listed, the internal energy is calculated from the definition of the enthalpy as ...
Heat and Thermodynamics 300 MCQ
... 17. Convection is a process of heat transfer that depends on 18. Which surface is the best radiator of heat 19. On a cloudiness night, the earth is gold because its heat is 20. The dimensional formula of K is 21. Thermal capacity of a good conductor is determined by 22. Thermal conductivity of a bad ...
... 17. Convection is a process of heat transfer that depends on 18. Which surface is the best radiator of heat 19. On a cloudiness night, the earth is gold because its heat is 20. The dimensional formula of K is 21. Thermal capacity of a good conductor is determined by 22. Thermal conductivity of a bad ...
Basic Concepts and Definitions
... very complicated. Hence, this approach is rarely employed but, has become more important in recent years. The behaviour of gas is to be described by summing up the behaviour of each molecule. In macroscopic approach the structure of matter is not considered, in fact it is simple, and only few variab ...
... very complicated. Hence, this approach is rarely employed but, has become more important in recent years. The behaviour of gas is to be described by summing up the behaviour of each molecule. In macroscopic approach the structure of matter is not considered, in fact it is simple, and only few variab ...
Chapter 20
... is not in an equilibrium state. One obvious example is an explosion. During an explosion, after it has started and before it ends, the exploding gas can not be described by a single temperature, pressure, and density, so it is not in an equilibrium state. The different parts of the exploding gas are ...
... is not in an equilibrium state. One obvious example is an explosion. During an explosion, after it has started and before it ends, the exploding gas can not be described by a single temperature, pressure, and density, so it is not in an equilibrium state. The different parts of the exploding gas are ...
Practice Test 2 Solutions Oct 2010 - University of KwaZulu
... • The B side is isothermal, thus we know that before and after B gets compressed the temperature remains the same • For the B side we know moles (n), new volume (VB2), temperature (TB2), and so we can find pressure (PB2) after the compression using PV = nRT • After the compres ...
... • The B side is isothermal, thus we know that before and after B gets compressed the temperature remains the same • For the B side we know moles (n), new volume (VB2), temperature (TB2), and so we can find pressure (PB2) after the compression using PV = nRT • After the compres ...
AP Lab - MW of Volatile Liquid - North Allegheny School District
... volume V of the pure gas at known temperature T and pressure P. The ideal gas law is PV = nRT, where n = moles of gas. But n = m/Mw where Mw equals molar mass. Therefore, Mw = mRT/PV. This method is applicable to any substance, provided the chemist has sufficient skill and sophisticated gas-handling ...
... volume V of the pure gas at known temperature T and pressure P. The ideal gas law is PV = nRT, where n = moles of gas. But n = m/Mw where Mw equals molar mass. Therefore, Mw = mRT/PV. This method is applicable to any substance, provided the chemist has sufficient skill and sophisticated gas-handling ...
fabrication of polymer form-silica nanocomposite thermal
... We have measured the phase diagram of several silicon alkoxide-CO2 binary systems and polymer-silicon alkoxide-CO2 ternary systems [1]. We have also explored preparation of polymer foam-silica nanocomposites by the above method in a batch reactor, and demonstrated that it is indeed to produce nanoco ...
... We have measured the phase diagram of several silicon alkoxide-CO2 binary systems and polymer-silicon alkoxide-CO2 ternary systems [1]. We have also explored preparation of polymer foam-silica nanocomposites by the above method in a batch reactor, and demonstrated that it is indeed to produce nanoco ...
Slides - fmeabj
... To understand the theory of the thermoelectric temperature measurement, especially thermocouples and develop some expertise in the measurement of temperature with thermocouples. To understand the concept of calibration and basic process of calibrating an instrument. Specifically, to realize the ...
... To understand the theory of the thermoelectric temperature measurement, especially thermocouples and develop some expertise in the measurement of temperature with thermocouples. To understand the concept of calibration and basic process of calibrating an instrument. Specifically, to realize the ...
Re-Evaluating Thermal Conductivity from the Top Down: Thermal
... Thermal diffusivity also depends on orientation of the crystal and, in minerals with solid-solutions, the proportions of endmembers in the mineral. For example, the clinopyroxene diopside (CaMgSi2O6) has a directionally averaged D of 3.4 mm2s-1 at 280 K, falling to 1.0 mm2 s-1 at 1000 K (figure 2). ...
... Thermal diffusivity also depends on orientation of the crystal and, in minerals with solid-solutions, the proportions of endmembers in the mineral. For example, the clinopyroxene diopside (CaMgSi2O6) has a directionally averaged D of 3.4 mm2s-1 at 280 K, falling to 1.0 mm2 s-1 at 1000 K (figure 2). ...
temperature 2015 10 13
... The basic algorithm of thermodynamics • (entropy) = log (number of sample points). • Entropy is additive. • When a constraint internal to an isolated system fixes an internal variable at a value x, the isolated system flips in a subset of quantum states. • Denote the number of quantum states in the ...
... The basic algorithm of thermodynamics • (entropy) = log (number of sample points). • Entropy is additive. • When a constraint internal to an isolated system fixes an internal variable at a value x, the isolated system flips in a subset of quantum states. • Denote the number of quantum states in the ...
KEMS448 Physical Chemistry Advanced Laboratory Work
... In addition to the decrease in vapour pressure, the raise in boiling point and the decrease of freezing point, the osmotic pressure is a colligative property. The solution is separated from a pure solvent with a semipermeable membrane, through which the solvent molecules can travel through, but the ...
... In addition to the decrease in vapour pressure, the raise in boiling point and the decrease of freezing point, the osmotic pressure is a colligative property. The solution is separated from a pure solvent with a semipermeable membrane, through which the solvent molecules can travel through, but the ...
[PDF]
... the zero thermal diffusivity is assumed reducing the equation to an ordinary differential equation, which can be solved when source term function q is assumed constant: ˆ � �� ) � �(�) ˆ � ...
... the zero thermal diffusivity is assumed reducing the equation to an ordinary differential equation, which can be solved when source term function q is assumed constant: ˆ � �� ) � �(�) ˆ � ...
Phase changes
... For the solid-liquid phase change, the slope of the equilibrium curve in the p-T diagram is so high that it is usually assumed that the temperature of fusion is the same at every pressure (this is used to approximate the triple point by the normal melting-point temperature and its corresponding vapo ...
... For the solid-liquid phase change, the slope of the equilibrium curve in the p-T diagram is so high that it is usually assumed that the temperature of fusion is the same at every pressure (this is used to approximate the triple point by the normal melting-point temperature and its corresponding vapo ...
Fundamentals
of
Physics
in
Engineering
I
PROBLEMES
PROPOSED
... 3.-The standard temperature and pressure (STP) is a state of an ideal gas with a temperature of 0ºC = 273.15 K and a pressure of 1 atm = 1.013 x 105 Pa. What would be the volume of a container that contains one mole of an ideal gas in a room at STP? 4.-A mixture of air and vaporized gasoline is comp ...
... 3.-The standard temperature and pressure (STP) is a state of an ideal gas with a temperature of 0ºC = 273.15 K and a pressure of 1 atm = 1.013 x 105 Pa. What would be the volume of a container that contains one mole of an ideal gas in a room at STP? 4.-A mixture of air and vaporized gasoline is comp ...
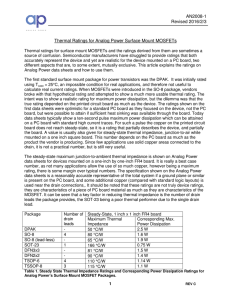
Thermal Ratings of Surface Mount Packages
... using Tcase = 25°C, an impossible condition for real applications, and therefore not useful to calculate real current ratings. When MOSFETs were introduced in the SO-8 package, vendors broke with that hypothetical rating and attempted to show a much more usable thermal rating. The intent was to show ...
... using Tcase = 25°C, an impossible condition for real applications, and therefore not useful to calculate real current ratings. When MOSFETs were introduced in the SO-8 package, vendors broke with that hypothetical rating and attempted to show a much more usable thermal rating. The intent was to show ...
Temperature Scales Temperature Scales
... - Figure 8.15 presents some common arrangement for establishing the reference temperature. 8.15a is necessary if the binding posts at the voltage-measuring instrument were at different temperatures, while the connection in 8.15b is satisfactory if the binding posts were at the same temperature. ...
... - Figure 8.15 presents some common arrangement for establishing the reference temperature. 8.15a is necessary if the binding posts at the voltage-measuring instrument were at different temperatures, while the connection in 8.15b is satisfactory if the binding posts were at the same temperature. ...
THERMAL ANALYSIS
... Also increased efficiency of analysis & increased accuracy. e) DYNAMIC MECHANICAL ANALYSIS Here the mechanical response of a sample is measured as it is deformed under oscillating load against temp/time. Here, Dynamic Modulus and /or damping of a substance under oscillatery load is measured. f ...
... Also increased efficiency of analysis & increased accuracy. e) DYNAMIC MECHANICAL ANALYSIS Here the mechanical response of a sample is measured as it is deformed under oscillating load against temp/time. Here, Dynamic Modulus and /or damping of a substance under oscillatery load is measured. f ...
Problems and Solutions
... formula given in the text of the problem should not be applied to the mixture of the saturated vapors in the bubbles formed on the surface separating the liquids. However, the numerical data have been chosen in such a way that even such incorrect solution of the problem gives the correct value of th ...
... formula given in the text of the problem should not be applied to the mixture of the saturated vapors in the bubbles formed on the surface separating the liquids. However, the numerical data have been chosen in such a way that even such incorrect solution of the problem gives the correct value of th ...
Table of Content
... below atmospheric (and at high temperatures), and, therefore, constitutes a limiting case. As we know, substances exist also in other forms: solids, liquids, etc. Also more often than not, in practice (as in process plants) gases (as well as other phases) may exist at substantially higher pressures ...
... below atmospheric (and at high temperatures), and, therefore, constitutes a limiting case. As we know, substances exist also in other forms: solids, liquids, etc. Also more often than not, in practice (as in process plants) gases (as well as other phases) may exist at substantially higher pressures ...










![[PDF]](http://s1.studyres.com/store/data/008813344_1-6b54197619c7ffbc0fcfc4fbb7e270fc-300x300.png)