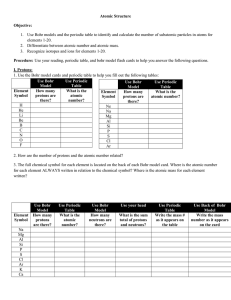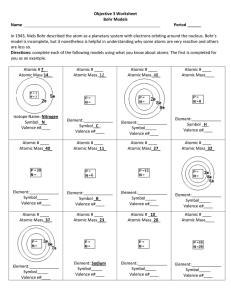
Objective 3 Worksheet Bohr Models Name Period In 1943, Niels
... Objective 3 Worksheet Bohr Models Name ...
... Objective 3 Worksheet Bohr Models Name ...
1 - PTO
... particular about how many beds are being used when guests are staying in the rooms. According to management, guests must spread out and fill each bed in the room before they can double up in a bed. One guest must be in every bed before a second guest can join them. Remember, extra guests are not all ...
... particular about how many beds are being used when guests are staying in the rooms. According to management, guests must spread out and fill each bed in the room before they can double up in a bed. One guest must be in every bed before a second guest can join them. Remember, extra guests are not all ...
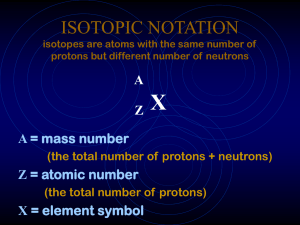
ISOTOPIC NOTATION isotopes are atoms with the same number
... 2. Give the complete chemical notation for the nuclide with 23 protons, 26 neutrons and 20 electrons. 49V3+ ...
... 2. Give the complete chemical notation for the nuclide with 23 protons, 26 neutrons and 20 electrons. 49V3+ ...
Atomic
... • Because the number of positive charges equals the number of negative charges, the atom is neutral (no charge) ...
... • Because the number of positive charges equals the number of negative charges, the atom is neutral (no charge) ...
Unit C3, C3.1
... the atomic structure of elements was unknown. Mendeleev tried to arrange the elements in a meaningful way based on their chemical reactions. First he put the elements in order of their increasing atomic weight. He then put elements with similar properties in the same column. However, he left gaps, a ...
... the atomic structure of elements was unknown. Mendeleev tried to arrange the elements in a meaningful way based on their chemical reactions. First he put the elements in order of their increasing atomic weight. He then put elements with similar properties in the same column. However, he left gaps, a ...
Ch 4 Powerpoint
... Determining subatomic particles in elements (GENERIC) Element symbol (X)one/two/three letter symbol for element Atomic number (Z) number of protons found in the element Mass number (A)number of protons + the number of neutrons The periodic table will give you all the information you need in order ...
... Determining subatomic particles in elements (GENERIC) Element symbol (X)one/two/three letter symbol for element Atomic number (Z) number of protons found in the element Mass number (A)number of protons + the number of neutrons The periodic table will give you all the information you need in order ...
1 - Atomic Theory - Crestwood Local Schools
... element that maintains the properties of that element. ...
... element that maintains the properties of that element. ...
sample
... Books, birds, buildings and all the other things around you are made from tiny things called atoms. You are made from atoms, too. Atoms are so small that a million of the biggest ones would make a pile less than half a millimeter high. We cannot see atoms, even with a microscope. It can be difficult ...
... Books, birds, buildings and all the other things around you are made from tiny things called atoms. You are made from atoms, too. Atoms are so small that a million of the biggest ones would make a pile less than half a millimeter high. We cannot see atoms, even with a microscope. It can be difficult ...
Chemistry
... 2000 years later Dalton proposed atomic theory (performed experimental science) Summarize Dalton’s atomic theory states: Elements made up of submicroscopic indivisible particles called atoms Atoms of same element identical Atoms of different elements are different Atoms of different elements can phy ...
... 2000 years later Dalton proposed atomic theory (performed experimental science) Summarize Dalton’s atomic theory states: Elements made up of submicroscopic indivisible particles called atoms Atoms of same element identical Atoms of different elements are different Atoms of different elements can phy ...
Sub Unit Plan 1 Chem Periodic Table
... II.3 Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. (3.1v) II.4 Elements can be differentiated by their physical properties. Physical properties of substances, such as density, conductivity, ...
... II.3 Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. (3.1v) II.4 Elements can be differentiated by their physical properties. Physical properties of substances, such as density, conductivity, ...
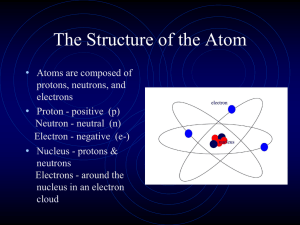
The Structure of the Atom
... 6. ________________ number. The mass of the atom is so small that there is a measure called the atomic 7. ________________ unit designated by amu. 8. ________________ and 9. ________________ make up the nucleus and are made up of 10. ________________. There are 11. ________________ uniquely differen ...
... 6. ________________ number. The mass of the atom is so small that there is a measure called the atomic 7. ________________ unit designated by amu. 8. ________________ and 9. ________________ make up the nucleus and are made up of 10. ________________. There are 11. ________________ uniquely differen ...
Chemistry
... 1) The smallest particle of an element that retains the properties of that element is the: a) cell b) proton c) electron d) neutron e) none of the above 2) Which of the following is not a part of Dalton’s atomic theory? a) All elements are composed of atoms. b) The positive charge of the atom is loc ...
... 1) The smallest particle of an element that retains the properties of that element is the: a) cell b) proton c) electron d) neutron e) none of the above 2) Which of the following is not a part of Dalton’s atomic theory? a) All elements are composed of atoms. b) The positive charge of the atom is loc ...
Name: Date:______ Period:____ Isotopes and Atomic Mass
... Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a physical basis for this idea by showing that certain substances could not be further broken down by chemical methods. During the late 19th and early 20th centuries, physicists discovered subatomic components and struc ...
... Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a physical basis for this idea by showing that certain substances could not be further broken down by chemical methods. During the late 19th and early 20th centuries, physicists discovered subatomic components and struc ...
Build An Atom - ChemConnections
... b. Whether an atom is neutral or an ion (cation or anion) and its respective charge. c. Orbits versus clouds. d. The total mass of an atom or ion. e. The mass relationship of isotopes and their rela ...
... b. Whether an atom is neutral or an ion (cation or anion) and its respective charge. c. Orbits versus clouds. d. The total mass of an atom or ion. e. The mass relationship of isotopes and their rela ...
Unit 3 Notes, Practice, and Review
... 19. The atomic number is unique for every element. It also tells the number of protons in that element. Every element on the periodic table has a unique number of protons. It’s like an element’s Social Security Number. 20. Atomic number is the number of protons and electrons in an atom. To get the n ...
... 19. The atomic number is unique for every element. It also tells the number of protons in that element. Every element on the periodic table has a unique number of protons. It’s like an element’s Social Security Number. 20. Atomic number is the number of protons and electrons in an atom. To get the n ...
Atom - Malibu High School
... and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a charged particle; # protons ≠ # electrons Electrons occupy most of the volume of an atom outside/around the nucleus. ...
... and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a charged particle; # protons ≠ # electrons Electrons occupy most of the volume of an atom outside/around the nucleus. ...
Help us improve Wikipedia by supporting it financially
... still have national names, and those which do not use the Latin alphabet cannot be expected to use the IUPAC name. According to IUPAC, the full name of an element is not capitalized, even if it is derived from a proper noun such as the elements californium or einsteinium (unless it would be capitali ...
... still have national names, and those which do not use the Latin alphabet cannot be expected to use the IUPAC name. According to IUPAC, the full name of an element is not capitalized, even if it is derived from a proper noun such as the elements californium or einsteinium (unless it would be capitali ...
1b Atomic Structure
... losing a single electron rather than stealing seven electrons. Hence, sodium atoms lose and electron, leaving them with 10 electrons and 11 protons. Doing the math, this means the total charge on sodium ion would be (-10 + +11 = +1), and is written as Na+1. ...
... losing a single electron rather than stealing seven electrons. Hence, sodium atoms lose and electron, leaving them with 10 electrons and 11 protons. Doing the math, this means the total charge on sodium ion would be (-10 + +11 = +1), and is written as Na+1. ...
Isotopes - Ms. Bergman`s Classes at DCIS Montbello
... Fill in Data Table 2 using the math in steps 1-5 from your analysis directions. ...
... Fill in Data Table 2 using the math in steps 1-5 from your analysis directions. ...
Review for Test
... 4. Describe the effects a stronger magnet would have had on the television image. __________________________________________________________________________________________________ __________________________________________________________________________________________________ ____________________ ...
... 4. Describe the effects a stronger magnet would have had on the television image. __________________________________________________________________________________________________ __________________________________________________________________________________________________ ____________________ ...
ISOTOPIC NOTATION isotopes are atoms with the same number of
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
Name
... • The mass number is the total number of protons and neutrons that make up the nucleus of an isotope. Designating Isotopes • Hyphen notation: The mass number is written with a hyphen after the name of the element. • uranium-235 • Nuclear symbol: The superscript indicates the mass number and the subs ...
... • The mass number is the total number of protons and neutrons that make up the nucleus of an isotope. Designating Isotopes • Hyphen notation: The mass number is written with a hyphen after the name of the element. • uranium-235 • Nuclear symbol: The superscript indicates the mass number and the subs ...
Lawrencium

Lawrencium is a synthetic chemical element with chemical symbol Lr (formerly Lw) and atomic number 103. It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radioactive metal, lawrencium is the eleventh transuranic element and is also the final member of the actinide series. Like all elements with atomic number over 100, lawrencium can only be produced in particle accelerators by bombarding lighter elements with charged particles. Twelve isotopes of lawrencium are currently known; the most stable is 266Lr with a half-life of 11 hours, but the shorter-lived 260Lr (half-life 2.7 minutes) is most commonly used in chemistry because it can be produced on a larger scale.Chemistry experiments have confirmed that lawrencium indeed behaves as a heavier homolog to lutetium in the periodic table, and is a trivalent element. It thus could also be classified as the first of the 7th-period transition metals: however, its electron configuration is anomalous for its position in the periodic table, having an s2p configuration instead of the s2d configuration of its homolog lutetium. This means that lawrencium may be less volatile than expected for its position in the periodic table and have a volatility comparable to that of lead.In the 1950s, 1960s, and 1970s, many claims of the synthesis of lawrencium of varying quality were made from laboratories in the Soviet Union and the United States. The priority of the discovery and therefore the naming of the element was disputed between Soviet and American scientists, and while the International Union of Pure and Applied Chemistry (IUPAC) established lawrencium as the official name for the element and gave the American team credit for the discovery, this was reevaluated in 1997, giving both teams shared credit for the discovery but not changing the element's name.