Quaternary Neptunium Compounds: Syntheses and
... have been synthesized by the reaction of Np, Cu or Ag, S, and K2S or Rb2S3 or Cs2S3 at 793 K (Rb) or 873 K. These isostructural compounds crystallize as black rectangular plates in the KCuZrS3 structure type in space group Cmcm of the orthorhombic system. The structure comprises MS4 (M=Cu or Ag) tet ...
... have been synthesized by the reaction of Np, Cu or Ag, S, and K2S or Rb2S3 or Cs2S3 at 793 K (Rb) or 873 K. These isostructural compounds crystallize as black rectangular plates in the KCuZrS3 structure type in space group Cmcm of the orthorhombic system. The structure comprises MS4 (M=Cu or Ag) tet ...
The Alkaline Earth Metals (Group 2) - Chemwiki
... Higher lattice energies cause the alkaline earth metals to be more reactive than the alkali metals toward group 15 elements. When heated, all alkaline earth metals, except for beryllium, react directly with carbon to form ionic carbides with the general formula MC2. The most important alkaline earth ...
... Higher lattice energies cause the alkaline earth metals to be more reactive than the alkali metals toward group 15 elements. When heated, all alkaline earth metals, except for beryllium, react directly with carbon to form ionic carbides with the general formula MC2. The most important alkaline earth ...
InorgCh8.2
... i. Unstable because Si—Si (340 kJ/mol) weaker than C—C (368 kJ/mol) ii. Si less electronegative than H (1.90 vs. 2.20) so attacked by Nu iii. Si larger than C, which also makes it more easily attacked by Nu iv. Empty d-orbitals act as e-pair acceptors ...
... i. Unstable because Si—Si (340 kJ/mol) weaker than C—C (368 kJ/mol) ii. Si less electronegative than H (1.90 vs. 2.20) so attacked by Nu iii. Si larger than C, which also makes it more easily attacked by Nu iv. Empty d-orbitals act as e-pair acceptors ...
Metalloid Al- and Ga-clusters: a novel dimension in organometallic
... clusters.2 Such clusters contain more MM contacts than ML (metal–ligand) bonds and mostly show similarities with respect to the topology to the arrangements of atoms in the elements themselves. Though most investigations on metalloid cluster species have been, and are still, performed in the field of ...
... clusters.2 Such clusters contain more MM contacts than ML (metal–ligand) bonds and mostly show similarities with respect to the topology to the arrangements of atoms in the elements themselves. Though most investigations on metalloid cluster species have been, and are still, performed in the field of ...
The d- and f- Block Element Block Elements The d- and f
... Due to an increase in nuclear charge which accompanies the filling of the inner d orbitals, there is an increase in ionisation enthalpy along each series of the transition elements from left to right. However, many small variations occur. Table 8.2 gives the values for the first three ionisation ent ...
... Due to an increase in nuclear charge which accompanies the filling of the inner d orbitals, there is an increase in ionisation enthalpy along each series of the transition elements from left to right. However, many small variations occur. Table 8.2 gives the values for the first three ionisation ent ...
Chapter 23 Metals and Metallurgy
... • Many important metals are included in this group. • Comprised of elements in d block of periodic table. ...
... • Many important metals are included in this group. • Comprised of elements in d block of periodic table. ...
Chemistry
... as 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique ...
... as 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique ...
1 chemistry of the nonmetals
... Each year between 75 and 80 quads, or quadrillion (1015) BTU (British thermal units), of energy is consumed in the United States.1 Less than 10% of this energy is provided by nuclear, solar, geothermal, or hydro power. The rest can be traced to a combustion reaction in which a fuel is oxidized by O2 ...
... Each year between 75 and 80 quads, or quadrillion (1015) BTU (British thermal units), of energy is consumed in the United States.1 Less than 10% of this energy is provided by nuclear, solar, geothermal, or hydro power. The rest can be traced to a combustion reaction in which a fuel is oxidized by O2 ...
chm 205 - National Open University of Nigeria
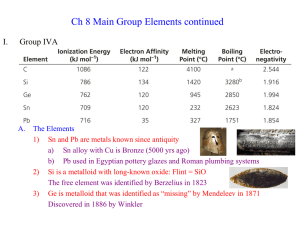
... elements of Group 13 show some irregularities. It was also pointed out that the first element in each group shows some anomalous behaviour. Now we extend our study to another group of p-block elements, namely, Group 4, which consists of carbon, silicon, germanium, tin and lead. This is the first gro ...
... elements of Group 13 show some irregularities. It was also pointed out that the first element in each group shows some anomalous behaviour. Now we extend our study to another group of p-block elements, namely, Group 4, which consists of carbon, silicon, germanium, tin and lead. This is the first gro ...
Chemistry (SPA)
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
Group 1: The Alkali Metals
... Properties and Facts about Alkali Metals Alkali metals are known for being some of the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and often have an oxidation state of +1. These metals are charac ...
... Properties and Facts about Alkali Metals Alkali metals are known for being some of the most reactive metals. This is due in part to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and often have an oxidation state of +1. These metals are charac ...
5073 Chemistry (SPA)
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
5073 Chemistry IGCSE ordinary level for 2016
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
Chemistry Revision Checklist F4 2017 (inc F3)
... Place in order of reactivity: potassium, sodium, calcium, magnesium, zinc, iron, (hydrogen) and copper, by reference to the reactions, if any, of the metals with: – water or steam – dilute hydrochloric acid and the reduction of their oxides with carbon Describe the reactivity series as related to th ...
... Place in order of reactivity: potassium, sodium, calcium, magnesium, zinc, iron, (hydrogen) and copper, by reference to the reactions, if any, of the metals with: – water or steam – dilute hydrochloric acid and the reduction of their oxides with carbon Describe the reactivity series as related to th ...
Metallic and nonmetallic double perovskites: A case study of A $ _2
... binding models suggest that the Re-Re hopping is the dominant effect [14]. Such a hopping is not unreasonable, given that we are dealing with t2g orbitals which point from one Re to another (along the face diagonal of the cubic perovskite structure) and the 5d orbitals which are spatially extended [ ...
... binding models suggest that the Re-Re hopping is the dominant effect [14]. Such a hopping is not unreasonable, given that we are dealing with t2g orbitals which point from one Re to another (along the face diagonal of the cubic perovskite structure) and the 5d orbitals which are spatially extended [ ...
Descriptive Chemistry of Elements p
... In 1985, a team of scientists discovered the third allotropic form of carbon called Buckminster fullerene (C60). There are other forms of carbon such as coke, charcoal and lamp black which are referred to as amorphous carbon. Coke is the residue left in the conversion of coal to coal gas. Charcoal i ...
... In 1985, a team of scientists discovered the third allotropic form of carbon called Buckminster fullerene (C60). There are other forms of carbon such as coke, charcoal and lamp black which are referred to as amorphous carbon. Coke is the residue left in the conversion of coal to coal gas. Charcoal i ...
TOPIC 12. THE ELEMENTS
... In 1869 Mendeleev proposed a classification of the then known 65 elements which placed priority on allocating elements to each family on the basis of similar properties with special emphasis on valence rather than atomic weight alone. He left blanks in families where discrepancies would otherwise ap ...
... In 1869 Mendeleev proposed a classification of the then known 65 elements which placed priority on allocating elements to each family on the basis of similar properties with special emphasis on valence rather than atomic weight alone. He left blanks in families where discrepancies would otherwise ap ...
WIPO IPC: Internet Publication
... o Non-metals: H, B, C, Si, N, P, O, S, Se, Te, noble gases, halogens o Metals: elements other than non-metals o Transition elements: elements with atomic numbers 21 to 30 inclusive, 39 to 48 inclusive, 57 to 80 inclusive, 89 upwards Section C covers : o pure chemistry, which covers inorganic compoun ...
... o Non-metals: H, B, C, Si, N, P, O, S, Se, Te, noble gases, halogens o Metals: elements other than non-metals o Transition elements: elements with atomic numbers 21 to 30 inclusive, 39 to 48 inclusive, 57 to 80 inclusive, 89 upwards Section C covers : o pure chemistry, which covers inorganic compoun ...
A millennial overview of transition metal chemistry
... The only well-known area of biometallic chemistry that seems to me to be unequivocally non-Wernerian, in a basic chemical sense, is the chemistry of the ferredoxins of the 2-, 3- and 4-iron types.18 Here we have polynuclear complexes in which electronic coupling of the metal atoms through the bridgi ...
... The only well-known area of biometallic chemistry that seems to me to be unequivocally non-Wernerian, in a basic chemical sense, is the chemistry of the ferredoxins of the 2-, 3- and 4-iron types.18 Here we have polynuclear complexes in which electronic coupling of the metal atoms through the bridgi ...
Module-2-s-and-d-elements - Львівський національний медичний
... conversion of the white lead used in the paintings to lead sulfide. The hydrogen peroxide oxidizes the black lead sulfide to white lead sulfate. It is used also as a source of oxygen in the fuel mixture for many rockets and torpedoes. ...
... conversion of the white lead used in the paintings to lead sulfide. The hydrogen peroxide oxidizes the black lead sulfide to white lead sulfate. It is used also as a source of oxygen in the fuel mixture for many rockets and torpedoes. ...
P-BLOCK ELEMENTS
... Cause: The presence of additional 10 d electrons in gallium offer very poor screening effect for the valence electrons. As a result effective nuclear charge increases and electron cloud shrinks. There is very small increase in atomic radius from Indium to thalium due to very poor shielding of f-elec ...
... Cause: The presence of additional 10 d electrons in gallium offer very poor screening effect for the valence electrons. As a result effective nuclear charge increases and electron cloud shrinks. There is very small increase in atomic radius from Indium to thalium due to very poor shielding of f-elec ...
Gmelin Tips and Reminders
... • Not all substances have a structure associated. Only compounds or compound parts, which are formed by discrete molecules or ions, have a structure. Compounds with a solid-state structure will not have a structure in the Gmelin database. If your search by structure brings up 0 hits, try the Search ...
... • Not all substances have a structure associated. Only compounds or compound parts, which are formed by discrete molecules or ions, have a structure. Compounds with a solid-state structure will not have a structure in the Gmelin database. If your search by structure brings up 0 hits, try the Search ...
CHAPTER 1
... and their relation to energy 4. Analytical chemistry—the identification of the components and composition of materials 5. Biochemistry—the study of substances and processes occurring in living things 6. Theoretical chemistry—the use of mathematics and computers to understand the principles behind ...
... and their relation to energy 4. Analytical chemistry—the identification of the components and composition of materials 5. Biochemistry—the study of substances and processes occurring in living things 6. Theoretical chemistry—the use of mathematics and computers to understand the principles behind ...
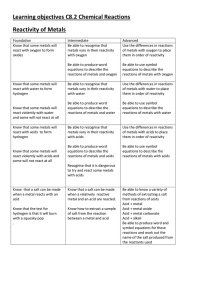
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... of a metal affects what it’s uses are ...
... of a metal affects what it’s uses are ...
Chapter 1: Matter and Change
... amount of matter that is present. Such properties include volume, mass, and the amount of energy in a substance. In contrast, intensive properties do not depend on the amount of matter present. Such properties include the melting point, boiling point, density, and ability to conduct electricity and ...
... amount of matter that is present. Such properties include volume, mass, and the amount of energy in a substance. In contrast, intensive properties do not depend on the amount of matter present. Such properties include the melting point, boiling point, density, and ability to conduct electricity and ...