Rapid Ether and Alcohol CO Bond Hydrogenolysis Catalyzed by
... to hydrocarbons present a major challenge in biomass research.2 Among the strategies for catalytic biomass hydrodeoxygenation,3 multifunctional catalyst systems consisting of a supported hydrogenation catalyst and an acid,4 either homogeneous5 or supported,6 have been studied. In addition to mineral ...
... to hydrocarbons present a major challenge in biomass research.2 Among the strategies for catalytic biomass hydrodeoxygenation,3 multifunctional catalyst systems consisting of a supported hydrogenation catalyst and an acid,4 either homogeneous5 or supported,6 have been studied. In addition to mineral ...
Fulltext PDF
... run reactions under mild conditions, we use ruthenium. They are the two wings of the same angel. The angel can’t fly if you clip either one.” N-Heterocyclic Carbenes, The Stable Carbenes Phosphine ligands are a very important class of molecules widely used in the synthesis of coordination and organo ...
... run reactions under mild conditions, we use ruthenium. They are the two wings of the same angel. The angel can’t fly if you clip either one.” N-Heterocyclic Carbenes, The Stable Carbenes Phosphine ligands are a very important class of molecules widely used in the synthesis of coordination and organo ...
Oxidation and Reduction Reactions
... consider to be a reduction reaction – the nucleophilic addition of hydrogen to a carbonyl (using NaBH4 or LiAlH4 as the source of nucleophilic hydrogen). This was a chemoselective reaction – in other words, the reducing agent only reduced one functional group (the carbonyl) and left others alone (e. ...
... consider to be a reduction reaction – the nucleophilic addition of hydrogen to a carbonyl (using NaBH4 or LiAlH4 as the source of nucleophilic hydrogen). This was a chemoselective reaction – in other words, the reducing agent only reduced one functional group (the carbonyl) and left others alone (e. ...
Lecture 1: Key Concepts in Stereoselective Synthesis
... Chiral boronate ester products of alkene hydrobration are useful synthetic intermediates. These substrates can be formed either using chiral reagents or asymmetric catalysis. Catalytic hydroboration and subsequent oxidation to the alcohol species remained as one of the most common reaction sequences ...
... Chiral boronate ester products of alkene hydrobration are useful synthetic intermediates. These substrates can be formed either using chiral reagents or asymmetric catalysis. Catalytic hydroboration and subsequent oxidation to the alcohol species remained as one of the most common reaction sequences ...
Document
... Applications: The Oxidation of Ethanol • In the body, ingested ethanol is oxidized in the liver first to CH3CHO (acetaldehyde), and then to CH3COO¯ (the acetate anion). • This oxidation is catalyzed by alcohol dehydrogenase. • If more ethanol is ingested than can be metabolized, the concentration o ...
... Applications: The Oxidation of Ethanol • In the body, ingested ethanol is oxidized in the liver first to CH3CHO (acetaldehyde), and then to CH3COO¯ (the acetate anion). • This oxidation is catalyzed by alcohol dehydrogenase. • If more ethanol is ingested than can be metabolized, the concentration o ...
New L-Serine Derivative Ligands as Cocatalysts for Diels
... It is noteworthy that ligand 8 completely deactivated the metal catalyst (entries 1, 2, and 3), with the strong catalytic effect of AlCl3 and FeCl3 (entries 2 and 4, Table 1) being totally suppressed. This suggests that the cation coordinates to the basic amine group of 6 and the resulting complex i ...
... It is noteworthy that ligand 8 completely deactivated the metal catalyst (entries 1, 2, and 3), with the strong catalytic effect of AlCl3 and FeCl3 (entries 2 and 4, Table 1) being totally suppressed. This suggests that the cation coordinates to the basic amine group of 6 and the resulting complex i ...
Asymmetric Catalytic Aldol
... • However narrow substrate tolerance! • Two types of enzymatic catalysts that effect aldol ...
... • However narrow substrate tolerance! • Two types of enzymatic catalysts that effect aldol ...
Slide 1
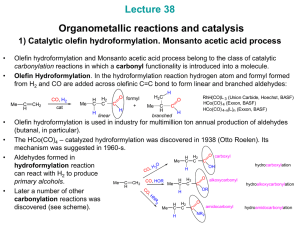
... Olefin hydroformylation and Monsanto acetic acid process belong to the class of catalytic carbonylation reactions in which a carbonyl functionality is introduced into a molecule. Olefin Hydroformylation. In the hydroformylation reaction hydrogen atom and formyl formed from H2 and CO are added across ...
... Olefin hydroformylation and Monsanto acetic acid process belong to the class of catalytic carbonylation reactions in which a carbonyl functionality is introduced into a molecule. Olefin Hydroformylation. In the hydroformylation reaction hydrogen atom and formyl formed from H2 and CO are added across ...
Asymmetry and Stereoisomers
... • the carbon atom with reference to valence number, bond strength, stability of carbon bonds with other elements and the formation of isomers to explain carbon compound diversity, including identification of chiral centres in optical isomers of simple organic compounds and distinction between cis- a ...
... • the carbon atom with reference to valence number, bond strength, stability of carbon bonds with other elements and the formation of isomers to explain carbon compound diversity, including identification of chiral centres in optical isomers of simple organic compounds and distinction between cis- a ...
Contents - Personal WWW Pages
... heterogeneous catalysis. i.e. faster reactions are often less selective. So, although homogeneous catalysis has a major advantage in the high selectivities which can be achieved, this is sometimes at the expense of lower reaction rates. Selectivity normally arises in homogenous catalysis because a s ...
... heterogeneous catalysis. i.e. faster reactions are often less selective. So, although homogeneous catalysis has a major advantage in the high selectivities which can be achieved, this is sometimes at the expense of lower reaction rates. Selectivity normally arises in homogenous catalysis because a s ...
Enantiodivergent conversion of chiral secondary alcohols into
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
Lecture 2
... Let AA be a multidentate ligand which must bond cis. For octahederal complex MAAbcde how many stereoisomers? ...
... Let AA be a multidentate ligand which must bond cis. For octahederal complex MAAbcde how many stereoisomers? ...
Materials Seminar Professor Carsten Sievers Georgia Institute of Technology
... with reduced reactivity and corrosiveness and increases the energy density of the product. In HDO, oxygen-containing functional groups are replaced by hydrogen, and water is formed as a by-product. This process can be performed over sulfided NiMo or CoMo catalysts. However, co-feeding of toxic H2S i ...
... with reduced reactivity and corrosiveness and increases the energy density of the product. In HDO, oxygen-containing functional groups are replaced by hydrogen, and water is formed as a by-product. This process can be performed over sulfided NiMo or CoMo catalysts. However, co-feeding of toxic H2S i ...
Module 1 Introduction to Heterogeneous Reactors and Application
... Leib, Pereira & Villadsden, NASCRE 1, Houston 2001 ...
... Leib, Pereira & Villadsden, NASCRE 1, Houston 2001 ...
pdf
... • Different enan)omers can have different effects on the body • Ac)ons of “Inac)ve Isomers” 1. One isomer possesses therapeu)c ac)on while the other contributes to ...
... • Different enan)omers can have different effects on the body • Ac)ons of “Inac)ve Isomers” 1. One isomer possesses therapeu)c ac)on while the other contributes to ...
Development of Novel Catalytic Asymmetric Reactions using
... a chemical intermediate. By proper tuning of the ligands and reaction conditions, we believed that the enolates should exhibit sufficient nucleophilicity to promote the aldol reaction with aldehydes under mild reaction conditions. At the time, little was known regarding the reactivity of Pd enolates ...
... a chemical intermediate. By proper tuning of the ligands and reaction conditions, we believed that the enolates should exhibit sufficient nucleophilicity to promote the aldol reaction with aldehydes under mild reaction conditions. At the time, little was known regarding the reactivity of Pd enolates ...
CHEMISTRY 1000
... consider to be a reduction reaction – the nucleophilic addition of hydrogen to a carbonyl (using NaBH4 or LiAlH4 as the source of nucleophilic hydrogen). This was a chemoselective reaction – in other words, the reducing agent only reduced one functional group (the carbonyl) and left others alone (e. ...
... consider to be a reduction reaction – the nucleophilic addition of hydrogen to a carbonyl (using NaBH4 or LiAlH4 as the source of nucleophilic hydrogen). This was a chemoselective reaction – in other words, the reducing agent only reduced one functional group (the carbonyl) and left others alone (e. ...
synthpp - Knockhardy
... When planning a synthetic route, chemists must consider... • the reagents required to convert one functional group into another • the presence of other functional groups - in case also they react ...
... When planning a synthetic route, chemists must consider... • the reagents required to convert one functional group into another • the presence of other functional groups - in case also they react ...
- Wiley Online Library
... copper salt 6. When the leaving group is not oxygen-based, salt metathesis with a metal alkoxide will convert 6 into copper alkoxide 7 from which copper(I) hydride 1 is regenerated by s-bond metathesis with a hydrosilane.[5] Alternatively, functionalization can occur through addition of copper inter ...
... copper salt 6. When the leaving group is not oxygen-based, salt metathesis with a metal alkoxide will convert 6 into copper alkoxide 7 from which copper(I) hydride 1 is regenerated by s-bond metathesis with a hydrosilane.[5] Alternatively, functionalization can occur through addition of copper inter ...
14. The Direct and Enantioselective Organocatalytic -Oxidation of Aldehydes
... important structural target for the development of new enantioselective technologies. At the present time, however, catalytic oxidation approaches to this asymmetric motif have relied exclusively upon the use of preformed enolates or enolate equivalents.1 As part of a program aimed at developing bro ...
... important structural target for the development of new enantioselective technologies. At the present time, however, catalytic oxidation approaches to this asymmetric motif have relied exclusively upon the use of preformed enolates or enolate equivalents.1 As part of a program aimed at developing bro ...
heterogeneous chiral catalyst derived from hydrolyzed
... and c, a chiral (asymmetric) centre is produced in (C abcd). In other words, it is a compound or group that has two enantiotopic atoms, faces or groups. For example, CH2XY has two enantiotopic H atoms. Asymmetric synthesis is only possible when the starting materials or conditions are optically acti ...
... and c, a chiral (asymmetric) centre is produced in (C abcd). In other words, it is a compound or group that has two enantiotopic atoms, faces or groups. For example, CH2XY has two enantiotopic H atoms. Asymmetric synthesis is only possible when the starting materials or conditions are optically acti ...
evans enolate alkylation
... This is critically important because the two enantiomers of the same compounds often/usually have very different properties when in come to biological activity. There are a number of different types of approaches to enantioselective synthesis. They include: 1) Substrate Control -A situation where a ...
... This is critically important because the two enantiomers of the same compounds often/usually have very different properties when in come to biological activity. There are a number of different types of approaches to enantioselective synthesis. They include: 1) Substrate Control -A situation where a ...
Catalytic Enantioselective Dibromination of Allylic Alcohols
... yields in most cases were moderate, diastereomeric dibromide products were not observed; starting material and malonate− alcohol transesterification products composed the majority of the remaining material. In the case of a p-vinyl substrate (entry 11), dibromination at the terminal olefin was not obs ...
... yields in most cases were moderate, diastereomeric dibromide products were not observed; starting material and malonate− alcohol transesterification products composed the majority of the remaining material. In the case of a p-vinyl substrate (entry 11), dibromination at the terminal olefin was not obs ...
The First Chiral Organometallic Triangle for Asymmetric Catalysis
... Coordination-driven self-assembly of inorganic and organometallic cycles and cages has witnessed tremendous growth over the past decade.1-5 Numerous metallocorners such as cis-[M(phosphine)2]2+,1 cis-[M(en)]2+,3 fac-(CO)3ReX,4 and M2(carboxylate)2 units5 have been utilized to construct metallosupram ...
... Coordination-driven self-assembly of inorganic and organometallic cycles and cages has witnessed tremendous growth over the past decade.1-5 Numerous metallocorners such as cis-[M(phosphine)2]2+,1 cis-[M(en)]2+,3 fac-(CO)3ReX,4 and M2(carboxylate)2 units5 have been utilized to construct metallosupram ...
Asymmetric hydrogenation

Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen preferentially to one of two faces of an unsaturated substrate molecule, such as an alkene or ketone. The selectivity derives from the manner that the substrate binds to the chiral catalysts. In jargon, this binding transmits spatial information (what chemists refer to as chirality) from the catalyst to the target, favoring the product as a single enantiomer. This enzyme-like selectivity is particularly applied to bioactive products such as pharmaceutical agents and agrochemicals.