catalysis lecture
... There are 5 types of reactions (and their reverse) which, in combination, account for most homogeneous catalytic cycles involving hydrocarbons. 1. Ligand Coordination and Dissociation 2. Insertion and Elimination 3. Nucleophilic attack on coordinated ligands 4. Oxidation and Reduction 5. Oxidative a ...
... There are 5 types of reactions (and their reverse) which, in combination, account for most homogeneous catalytic cycles involving hydrocarbons. 1. Ligand Coordination and Dissociation 2. Insertion and Elimination 3. Nucleophilic attack on coordinated ligands 4. Oxidation and Reduction 5. Oxidative a ...
Jan-Erling Bäckvall - The Scripps Research Institute
... Catalytic oxidation using environmentally benign terminal oxidants (O2, H2O2) is greatly facilitated by incorporation of electron transfer mediators into a catalytic system. Why? ...
... Catalytic oxidation using environmentally benign terminal oxidants (O2, H2O2) is greatly facilitated by incorporation of electron transfer mediators into a catalytic system. Why? ...
Organometallic Compounds and Catalysis: Synthesis
... Organometallic Compounds and Catalysis: Synthesis and Use of Wilkinson’s Catalyst Organometallic chemistry is the chemistry of compounds which contain a metal carbon bond. Research interest in this area is largely fueled by potential applications of organometallic compounds as catalysts in industria ...
... Organometallic Compounds and Catalysis: Synthesis and Use of Wilkinson’s Catalyst Organometallic chemistry is the chemistry of compounds which contain a metal carbon bond. Research interest in this area is largely fueled by potential applications of organometallic compounds as catalysts in industria ...
Chapter 7
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
Homogeneously catalysed hydrogenation of unsaturated fatty acids
... y = x + - a+bx This equation, which represents a hyperbola, allows a simple representation of a complicated chemical reaction and is of practical importance for a systematic study of catalytic processes. It was found that the course of the hydrogenation of unsaturated acids under influence of Cu- an ...
... y = x + - a+bx This equation, which represents a hyperbola, allows a simple representation of a complicated chemical reaction and is of practical importance for a systematic study of catalytic processes. It was found that the course of the hydrogenation of unsaturated acids under influence of Cu- an ...
molecules Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis
... R2 = Bn, Ph, 4-(MeO)C6H4, 4-MeC6H4, 4-PhC6H4, 4-FC6H4 ...
... R2 = Bn, Ph, 4-(MeO)C6H4, 4-MeC6H4, 4-PhC6H4, 4-FC6H4 ...
Chapter 7
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
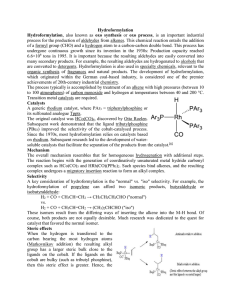
Hydroformylation Hydroformylation, also known as oxo synthesis or
... 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic s ...
... 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic s ...
File
... • As with phosphines, this allows bonding from carbene to metal (lone pair in sp2 orbital) and back-bonding (to empty p orbital) • Back bonding is significant in fisher and schrock carbenes but not with nucleophilic ...
... • As with phosphines, this allows bonding from carbene to metal (lone pair in sp2 orbital) and back-bonding (to empty p orbital) • Back bonding is significant in fisher and schrock carbenes but not with nucleophilic ...
- M E S KVM College Valanchery.
... Hydroformylation of alkenes may lead to alcohol and aldehyde products. Which statement is correct? The stereoselectivity of the reaction gives the aldehyde:alcohol ratio The chemoselectivity of the reaction gives the n:i aldehyde ratio The regioselectivity of the reaction gives the aldehyde:alcohol ...
... Hydroformylation of alkenes may lead to alcohol and aldehyde products. Which statement is correct? The stereoselectivity of the reaction gives the aldehyde:alcohol ratio The chemoselectivity of the reaction gives the n:i aldehyde ratio The regioselectivity of the reaction gives the aldehyde:alcohol ...
Chapter 7 - Alkenes and Alkynes I less substituted alkene due to
... - If there is an E2 elimination where there are two anticoplanar β hydrogens, the major product will be one that follows Zaitsev's rule 1 To determine the degree of substitution, look at both carbon atoms of the double bond. Count how many alkyl groups are attached to both carbon atoms total. 2 Make ...
... - If there is an E2 elimination where there are two anticoplanar β hydrogens, the major product will be one that follows Zaitsev's rule 1 To determine the degree of substitution, look at both carbon atoms of the double bond. Count how many alkyl groups are attached to both carbon atoms total. 2 Make ...
Chapter 7
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
... • In general, the preferential formation of one product because the free energy of activation is lower than another product, therefore, the rate of its formation is faster, is called Kinetic Control of Product Formation. ...
Postprint
... [IrCl(CO)(PPh3)2] (Figure 1.a), a catalyst of the [L3MX]-type .4 Following parallel development with other platinum group metals, Shrock and Osborn (Figure 1.d) discovered that an [L4Rh]+X- complex could convert in situ to an [L2Rh]+X- active catalyst. Overall having a 2:1 mono-dentate ligand/metal ...
... [IrCl(CO)(PPh3)2] (Figure 1.a), a catalyst of the [L3MX]-type .4 Following parallel development with other platinum group metals, Shrock and Osborn (Figure 1.d) discovered that an [L4Rh]+X- complex could convert in situ to an [L2Rh]+X- active catalyst. Overall having a 2:1 mono-dentate ligand/metal ...
Slides
... priority on the other carbon the double bond is Z (zusammen) è If the highest priority groups are on opposite sides the alkeneis E (entgegen) ...
... priority on the other carbon the double bond is Z (zusammen) è If the highest priority groups are on opposite sides the alkeneis E (entgegen) ...
i m. pharm. - Rajiv Gandhi University of Health Sciences
... transformed into various functionalities, without racemization, to synthesize industrially important chemicals such as pharmaceuticals, agrochemicals and natural products. The catalysts for the asymmetric reduction of ketones can be classified into two categories: chemical and biological methodologi ...
... transformed into various functionalities, without racemization, to synthesize industrially important chemicals such as pharmaceuticals, agrochemicals and natural products. The catalysts for the asymmetric reduction of ketones can be classified into two categories: chemical and biological methodologi ...
Synthetic route to novel asymmetric tetradentate ligands
... Vast interest has been developed by bioinorganic chemists to devise small molecule models, the properties of which can be compared with different metalloproteins [1]. Biomimetic modeling of metal complexes has denoted a better catalytic behaviour when the complex is able to easily coordinate the sub ...
... Vast interest has been developed by bioinorganic chemists to devise small molecule models, the properties of which can be compared with different metalloproteins [1]. Biomimetic modeling of metal complexes has denoted a better catalytic behaviour when the complex is able to easily coordinate the sub ...
Perspective and prospects for pincer ligand chemistry
... out if a catalyst is truly homogeneous or if a tethered pincer catalyst stays bound to the substrate. In the absence of such tests, any mechanistic proposals should be regarded with due caution. While some pincer ligand complexes have shown the ability to readily release the metal from the tridentat ...
... out if a catalyst is truly homogeneous or if a tethered pincer catalyst stays bound to the substrate. In the absence of such tests, any mechanistic proposals should be regarded with due caution. While some pincer ligand complexes have shown the ability to readily release the metal from the tridentat ...
Highly Enantioselective Cyclocarbonylation of Allylic
... We have carried out cyclocarbonylation of various aliphatic and aromatic unsaturated allylic alcohols catalyzed by Pd-BICP and Pd-Xyl-BICP complexes under the optimal reaction conditions (Table 1). In general, enantioselectivity in the cyclocarbonylation of several of the allylic alcohols with the P ...
... We have carried out cyclocarbonylation of various aliphatic and aromatic unsaturated allylic alcohols catalyzed by Pd-BICP and Pd-Xyl-BICP complexes under the optimal reaction conditions (Table 1). In general, enantioselectivity in the cyclocarbonylation of several of the allylic alcohols with the P ...
Microsoft Word
... isolated yield. Benzoic anhydride was also used as the acylating species to yield the corresponding benzoates. Several primary, secondary, tertiary, and b-substituted alcohols were acylated by using 5-10 mol% of magnesium bromide as the catalyst (Scheme 8). Scheme 8. ...
... isolated yield. Benzoic anhydride was also used as the acylating species to yield the corresponding benzoates. Several primary, secondary, tertiary, and b-substituted alcohols were acylated by using 5-10 mol% of magnesium bromide as the catalyst (Scheme 8). Scheme 8. ...
Lecture 17-edited
... 1,2-di > 1,2-tri > 1,2-tetra substituted. Different metal catalysts have been used for the purpose. Among them, platinum (Pt), iridium (Ir), ruthenium (Ru), rhodium (Rh), palladium (Pd) and nickel (Ni) catalysts are commonly used. Ni Catalysts: C. R. Sarko, M. DiMare, Encyclopedia of Reagents for Or ...
... 1,2-di > 1,2-tri > 1,2-tetra substituted. Different metal catalysts have been used for the purpose. Among them, platinum (Pt), iridium (Ir), ruthenium (Ru), rhodium (Rh), palladium (Pd) and nickel (Ni) catalysts are commonly used. Ni Catalysts: C. R. Sarko, M. DiMare, Encyclopedia of Reagents for Or ...
O V O O RO OH t-BuOOH, CH2Cl2, Ti(OPr-i)4(cat), 20 oC (L)
... These are not actually enantiomers of each other, but rather diastereomers, because the chirality of the C bearing the ethyl is the same in each case ((R)-). For the dihydroxylations, however, they function as the complementary systems to get enantiomeric products; the unofficial term pseudoenantiom ...
... These are not actually enantiomers of each other, but rather diastereomers, because the chirality of the C bearing the ethyl is the same in each case ((R)-). For the dihydroxylations, however, they function as the complementary systems to get enantiomeric products; the unofficial term pseudoenantiom ...
Asymmetric hydrogenation

Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen preferentially to one of two faces of an unsaturated substrate molecule, such as an alkene or ketone. The selectivity derives from the manner that the substrate binds to the chiral catalysts. In jargon, this binding transmits spatial information (what chemists refer to as chirality) from the catalyst to the target, favoring the product as a single enantiomer. This enzyme-like selectivity is particularly applied to bioactive products such as pharmaceutical agents and agrochemicals.