IV. The Transmission Electron Microscope
... The sputter ion pump works by ionizing gas that falls within a magnetically confined cold cathode, then reacting these with (or burying them in) plates of titanium. The ion pump consists of two parallel plates (3) of negatively-charged titanium (cathode) and cylindrical anodes (4) all surrounded by ...
... The sputter ion pump works by ionizing gas that falls within a magnetically confined cold cathode, then reacting these with (or burying them in) plates of titanium. The ion pump consists of two parallel plates (3) of negatively-charged titanium (cathode) and cylindrical anodes (4) all surrounded by ...
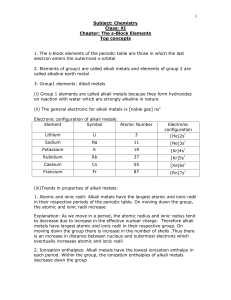
The s-Block Elements Top concepts 1. The s-block
... metals to higher energy levels. Excited state is quite unstable and therefore when these excited electrons come back to their original energy levels, they emit extra energy, which falls in the visible region of the electromagnetic spectrum and thus appear coloured. Characteristic flame colouration b ...
... metals to higher energy levels. Excited state is quite unstable and therefore when these excited electrons come back to their original energy levels, they emit extra energy, which falls in the visible region of the electromagnetic spectrum and thus appear coloured. Characteristic flame colouration b ...
VUV photochemistry of small biomolecules
... Muñoz Caro and co-workers (2002) have shown that the hydrochloric acid extract of the so-called ‘‘yellow-stuff’’, formed under UV irradiation of simulated interstellar ices in the laboratory, contains numerous AAs. Exploring the VUV photochemistry of these important biomolecules is of considerable ...
... Muñoz Caro and co-workers (2002) have shown that the hydrochloric acid extract of the so-called ‘‘yellow-stuff’’, formed under UV irradiation of simulated interstellar ices in the laboratory, contains numerous AAs. Exploring the VUV photochemistry of these important biomolecules is of considerable ...
Concentration Fluctuations and Capacitive
... electrode, and calculated analogously to ps. While cation− solvent and anion−solvent correlations do not exhibit strong compositional dependence, solvent-polarization correlations are enhanced with increasing ion concentration. The negative value of this covariance arises molecularly from swapping m ...
... electrode, and calculated analogously to ps. While cation− solvent and anion−solvent correlations do not exhibit strong compositional dependence, solvent-polarization correlations are enhanced with increasing ion concentration. The negative value of this covariance arises molecularly from swapping m ...
Gas Chromatography
... - radioactive decay-based detector - selective for compounds containing electronegative atoms, such as halogens Principle of Operation - based on the capture of electrons by electronegative atoms in a molecule - electrons are produced by ionization of the carrier gas with a radioactive source such a ...
... - radioactive decay-based detector - selective for compounds containing electronegative atoms, such as halogens Principle of Operation - based on the capture of electrons by electronegative atoms in a molecule - electrons are produced by ionization of the carrier gas with a radioactive source such a ...
Cold interactions between an Yb ion and a Li atom
... (equation of motion) within the coupled cluster singles, doubles, and linear triples framework (LRCC3) [32,33]. The basis set superposition error is corrected by using the counterpoise correction of Boys and Bernardi [34]. The CCSD(T) and RCCSD(T) calculations were performed with the MOLPRO suite of ...
... (equation of motion) within the coupled cluster singles, doubles, and linear triples framework (LRCC3) [32,33]. The basis set superposition error is corrected by using the counterpoise correction of Boys and Bernardi [34]. The CCSD(T) and RCCSD(T) calculations were performed with the MOLPRO suite of ...
Qualitative Analysis Test for Ions
... (b) Two of the compounds in the table produce a colour in a flame test. Give the name of one of these compounds and the colour it produces in the flame test. ...
... (b) Two of the compounds in the table produce a colour in a flame test. Give the name of one of these compounds and the colour it produces in the flame test. ...
2. Electrodics
... proportion (50%) by each species. To calculate the transference number one does not need the absolute mobility of an ion, but only the ratio between the two mobilities. The transference number is not constant with concentration, because the mobilities change with changing the concentration (due to i ...
... proportion (50%) by each species. To calculate the transference number one does not need the absolute mobility of an ion, but only the ratio between the two mobilities. The transference number is not constant with concentration, because the mobilities change with changing the concentration (due to i ...
What is the pH of a 0.100 M
... Kw = [H3O+][OH-] = 1.0 x 10-14 Le Chatelier: What happens if more H+ is added to water? What happens if more OH- is added to water? The equilibrium of water, H3O+ and OH- means if we know either the concentration of H3O+ or the concentration of OH- in an aqueous solution, then we know the concentrat ...
... Kw = [H3O+][OH-] = 1.0 x 10-14 Le Chatelier: What happens if more H+ is added to water? What happens if more OH- is added to water? The equilibrium of water, H3O+ and OH- means if we know either the concentration of H3O+ or the concentration of OH- in an aqueous solution, then we know the concentrat ...
(1–1.5 kV) nitrogen-ion bombardment on sharply pointed tips
... performed, at room temperature and 473 K, by applying a negative voltage to the tip in the presence of nitrogen gas ~1023 –1025 Torr! in an ultrahigh vacuum atom-probe field-ion microscope ~APFIM!. Tip sharpening, as a consequence of sputtering, is observed directly in situ via APFIM. This sharpenin ...
... performed, at room temperature and 473 K, by applying a negative voltage to the tip in the presence of nitrogen gas ~1023 –1025 Torr! in an ultrahigh vacuum atom-probe field-ion microscope ~APFIM!. Tip sharpening, as a consequence of sputtering, is observed directly in situ via APFIM. This sharpenin ...
Reactions of first-row transition metal ions with propargyl alcohol in
... M-O bond energies for Sc, Ti and V. Furthermore, the conjugation effect in PPA decreases the energy of C-OH and O-H. It can be presumed that these three ions are energetically favored to insert into C-OH and O-H, and the main products of these ions with PPA will be O-rich species, as confirmed by ou ...
... M-O bond energies for Sc, Ti and V. Furthermore, the conjugation effect in PPA decreases the energy of C-OH and O-H. It can be presumed that these three ions are energetically favored to insert into C-OH and O-H, and the main products of these ions with PPA will be O-rich species, as confirmed by ou ...
Discharge Generation of Atomic Iodine
... generation of atomic iodine, generation of singlet oxygen (excited oxygen molecule) as a source of energy for laser pumping, and efficient mixing of these compounds in the nozzle. Both atomic iodine and singlet oxygen can be produced chemically or in electric discharge. In our laboratory we have sta ...
... generation of atomic iodine, generation of singlet oxygen (excited oxygen molecule) as a source of energy for laser pumping, and efficient mixing of these compounds in the nozzle. Both atomic iodine and singlet oxygen can be produced chemically or in electric discharge. In our laboratory we have sta ...
A model based on equations of kinetics to study nitrogen dioxide
... pressure was observed. The creation of CO2 and N2 inside the reactor regardless of N2 temperature was reported. According to their results, the temperature decreases for combustion and the reduction of the conversion efficiency of NOx to NO increases during the plasma formation. It shows that, the c ...
... pressure was observed. The creation of CO2 and N2 inside the reactor regardless of N2 temperature was reported. According to their results, the temperature decreases for combustion and the reduction of the conversion efficiency of NOx to NO increases during the plasma formation. It shows that, the c ...
"Positron-impact ionization, positronium formation, and electronic excitation cross sections for diatomic molecules" Phys. Rev. A 72 (2005), 062713. J. P. Marler and C.M. Surko (PDF)
... using a technique that relies on the fact that the positron orbits are strongly magnetized 关9,10兴. In a strong magnetic field, namely, where the positron’s gyroradius is small compared to the characteristic dimensions of the scattering apparatus 共but still large compared to atomic dimensions兲, the t ...
... using a technique that relies on the fact that the positron orbits are strongly magnetized 关9,10兴. In a strong magnetic field, namely, where the positron’s gyroradius is small compared to the characteristic dimensions of the scattering apparatus 共but still large compared to atomic dimensions兲, the t ...
M - Science Skool!
... calculate just how much of each reactant they need, and how much useful product is likely to be made. In everyday life, the amount of something is usually its mass in grams if it is solid, or its volume in cubic centimetres if it is a liquid or gas. Other measures might be used for bigger quantities ...
... calculate just how much of each reactant they need, and how much useful product is likely to be made. In everyday life, the amount of something is usually its mass in grams if it is solid, or its volume in cubic centimetres if it is a liquid or gas. Other measures might be used for bigger quantities ...
Study of excited states of fluorinated copper phthalocyanine by inner
... to an unoccupied state which is distributed at the C–F bond in FCuPc molecule exists at hν = 691.2 eV and (ii) the transition dipole moment from F 1s to this unoccupied state is small. It is expected that a possible assignment of this unoccupied state is (C–F(s))∗ , since the transition from 1s to ...
... to an unoccupied state which is distributed at the C–F bond in FCuPc molecule exists at hν = 691.2 eV and (ii) the transition dipole moment from F 1s to this unoccupied state is small. It is expected that a possible assignment of this unoccupied state is (C–F(s))∗ , since the transition from 1s to ...
Chem 321 Lecture 11 - Chemical Activities
... concentration ([An]) arises because of ionic interactions between mobile ions in a solution. Individual ions in solution are surrounded by ions of opposite charge (they are shielded). Consequently, the formal charge an ion projects to other ions is less than it normally would be, so it interacts wit ...
... concentration ([An]) arises because of ionic interactions between mobile ions in a solution. Individual ions in solution are surrounded by ions of opposite charge (they are shielded). Consequently, the formal charge an ion projects to other ions is less than it normally would be, so it interacts wit ...
Does electrical double layer formation lead to salt exclusion or to
... charge. Such ions are called charge determing 共CD兲. Examples are protons 共H+兲 and hydroxyl ions 共OH−兲 for oxidic surfaces. They are administered as acids 共HNO3兲 and/or bases 共KOH兲. Automatically KNO3 may be formed. The concentration in the solution of these electrolytes is mostly low, say less than ...
... charge. Such ions are called charge determing 共CD兲. Examples are protons 共H+兲 and hydroxyl ions 共OH−兲 for oxidic surfaces. They are administered as acids 共HNO3兲 and/or bases 共KOH兲. Automatically KNO3 may be formed. The concentration in the solution of these electrolytes is mostly low, say less than ...
solute
... dissolved in water (aqueous) In order for a solution to carry an electrical current, it must contain ions that are free to move. ...
... dissolved in water (aqueous) In order for a solution to carry an electrical current, it must contain ions that are free to move. ...
CHEMISTRY NOTES – CHAPTERS 20 AND 21
... Step a would be the have the largest Ka. Each successive step would have a smaller Ka as it becomes increasingly difficult to remove a positive hydrogen ion from a more negative anion. The terms concentrated and dilute should not be confused with the terms strong and weak in relation to acids and ba ...
... Step a would be the have the largest Ka. Each successive step would have a smaller Ka as it becomes increasingly difficult to remove a positive hydrogen ion from a more negative anion. The terms concentrated and dilute should not be confused with the terms strong and weak in relation to acids and ba ...
Nanostructured Li Ion Insertion Electrodes. 1
... tool to monitor the fundamental changes in steady-state conditions, especially in cases where a macrohomogeneous scheme arises similar to, for instance, that depicted in Figure 1. Therefore, the electrode’s nanostructure is composed of two mixed phases: (i) a solid phase having the form of connected ...
... tool to monitor the fundamental changes in steady-state conditions, especially in cases where a macrohomogeneous scheme arises similar to, for instance, that depicted in Figure 1. Therefore, the electrode’s nanostructure is composed of two mixed phases: (i) a solid phase having the form of connected ...
Міністерство охорони здоров`я України
... The mass of a gas that dissolves at a constant temperature in a given volume of a liquid is directly proportional to the partial pressure of the gas. where C is the mass concentration of the gas in a saturated solution, p is the partial pressure, and k is a constant known as Henry's law constant (or ...
... The mass of a gas that dissolves at a constant temperature in a given volume of a liquid is directly proportional to the partial pressure of the gas. where C is the mass concentration of the gas in a saturated solution, p is the partial pressure, and k is a constant known as Henry's law constant (or ...
Charging of Oil-Water Interfaces Due to Spontaneous Adsorption of
... coalescence of the emulsion droplets. In order to increase the reliability of the data, two alternative procedures for emulsion preparation were used. The first procedure was close to the standard method for preparation of surfactant containing emulsions. The purified and presaturated liquid phases ...
... coalescence of the emulsion droplets. In order to increase the reliability of the data, two alternative procedures for emulsion preparation were used. The first procedure was close to the standard method for preparation of surfactant containing emulsions. The purified and presaturated liquid phases ...
Ion source

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.