Ultrafast Switching of Coherent Electronic Excitation
... studies on potassium dimers (K2) have been performed [25,26]. These studies confirm the applicability of strong field control by SPODS to molecules. In particular, the insights into the physical mechanism of strong field control obtained on atoms can be used to design shaped laser pulses to efficien ...
... studies on potassium dimers (K2) have been performed [25,26]. These studies confirm the applicability of strong field control by SPODS to molecules. In particular, the insights into the physical mechanism of strong field control obtained on atoms can be used to design shaped laser pulses to efficien ...
Dissociation of H in the energy region at the n state
... observed time-dependent Balmer-a intensity. In these experiments, states of ungerade symmetry are excited, while in the present experiments gerade states are accessed. These experimental results are therefore not directly applicable, but we can conclude that the observed Hq signal mainly results fro ...
... observed time-dependent Balmer-a intensity. In these experiments, states of ungerade symmetry are excited, while in the present experiments gerade states are accessed. These experimental results are therefore not directly applicable, but we can conclude that the observed Hq signal mainly results fro ...
Chemistry Lecture *34". Ionic. Compounds I-P one atom trans
... I-P one atom trans-Pers its electrons to another, they will stick together because one atom will have a positive charge and the other will have a negative charge. Electrostatic -Porce is the -Porce o£ attraction between opposite charges. Thus, anions and cations will stick together due to the electr ...
... I-P one atom trans-Pers its electrons to another, they will stick together because one atom will have a positive charge and the other will have a negative charge. Electrostatic -Porce is the -Porce o£ attraction between opposite charges. Thus, anions and cations will stick together due to the electr ...
Unit 6: Solution Chemistry Content Outline: Basic Solution Chemistry
... For example, cold tea is much harder to sweeten than hot tea. 3. Gases also require a specified pressure…remember that temperature directly affects pressure. For example, cold water holds more dissolved Oxygen gas than warm water. The amount of dissolved Oxygen gas determines the types of life forms ...
... For example, cold tea is much harder to sweeten than hot tea. 3. Gases also require a specified pressure…remember that temperature directly affects pressure. For example, cold water holds more dissolved Oxygen gas than warm water. The amount of dissolved Oxygen gas determines the types of life forms ...
Frequency, temperature and salinity variation of the
... XISTING systems for underwater communication largely depend on acoustic technologies. However, acoustic communication is riddled with problems including time-varying multipath propagation and large latencies. Therefore, Al-Shammaa et. al. claim that radio communication is a viable alternative [1]. T ...
... XISTING systems for underwater communication largely depend on acoustic technologies. However, acoustic communication is riddled with problems including time-varying multipath propagation and large latencies. Therefore, Al-Shammaa et. al. claim that radio communication is a viable alternative [1]. T ...
Three-dimensional depth profiling of molecular structures
... by sputtering cycles, where the surface is eroded by the ion beam. During these cycles, the fullerene projectile beam is operated in a dc mode and digitally rastered across the same field of view as used during data acquisition. This feature is important to note, since it is different from conventio ...
... by sputtering cycles, where the surface is eroded by the ion beam. During these cycles, the fullerene projectile beam is operated in a dc mode and digitally rastered across the same field of view as used during data acquisition. This feature is important to note, since it is different from conventio ...
mole concept - Gyan Vigyan Sarita
... Atomic Mass: The atomic masses of all the elements were obtained by comparing with the mass of hydrogen taken as 1 (because it was the lightest element). But by doing so, the atomic masses of most of the elements come out to be fractional. Hence, the reference was changed from hydrogen to oxygen tak ...
... Atomic Mass: The atomic masses of all the elements were obtained by comparing with the mass of hydrogen taken as 1 (because it was the lightest element). But by doing so, the atomic masses of most of the elements come out to be fractional. Hence, the reference was changed from hydrogen to oxygen tak ...
Solution
... In addition to the attractive Mz+-O- interaction noted above, we must recognize that a repulsive Mz+-H+ interaction also exists. In fact, we may go so far as to say that the hydration of a metal ion really involves two simultaneous processes: (a) attraction of the negative pole of the water molecu ...
... In addition to the attractive Mz+-O- interaction noted above, we must recognize that a repulsive Mz+-H+ interaction also exists. In fact, we may go so far as to say that the hydration of a metal ion really involves two simultaneous processes: (a) attraction of the negative pole of the water molecu ...
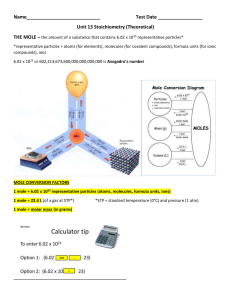
Unit 13 Stoichiometry (Theoretical)
... THE MOLE – the amount of a substance that contains 6.02 x 1023 representative particles* *representative particles = atoms (for elements), molecules (for covalent compounds), formula units (for ionic compounds), ions 6.02 x 1023 or 602,213,673,600,000,000,000,000 is Avogadro’s number ...
... THE MOLE – the amount of a substance that contains 6.02 x 1023 representative particles* *representative particles = atoms (for elements), molecules (for covalent compounds), formula units (for ionic compounds), ions 6.02 x 1023 or 602,213,673,600,000,000,000,000 is Avogadro’s number ...
Ligand field density functional theory calculation of the 4f²→ 4f¹5d¹
... DFT can in principle be used to calculate optical properties of materials,14–16 e.g. using TDDFT17,18 or Delta-SCF.19,20 However, combining classical ligand field theory21,22 or, in a certain context, crystal field theory with DFT gives rise to interesting results with a relatively good agreement wi ...
... DFT can in principle be used to calculate optical properties of materials,14–16 e.g. using TDDFT17,18 or Delta-SCF.19,20 However, combining classical ligand field theory21,22 or, in a certain context, crystal field theory with DFT gives rise to interesting results with a relatively good agreement wi ...
Fragmentation Dynamics of Small Molecules upon
... molecules [25, 26, 27] and small rare gas clusters [28] were conducted at the LCLS. These experiments revealed novel effects such as double or multiple core-hole creation and frustrated X-ray absorption [22, 25, 26, 27, 28], and showed that very high levels of ionization can be achieved (e.g., up to ...
... molecules [25, 26, 27] and small rare gas clusters [28] were conducted at the LCLS. These experiments revealed novel effects such as double or multiple core-hole creation and frustrated X-ray absorption [22, 25, 26, 27, 28], and showed that very high levels of ionization can be achieved (e.g., up to ...
Excersises and Study Guide
... term in this expression is the centrifugal barrier. Assume that a reaction will occur if this centrifugal barrier can be overcome. Thus, calculate the maximum impact parameter, which leads to orbiting of the colliding particles; e.g., at closest approach, the effective potential has to be zero. Then ...
... term in this expression is the centrifugal barrier. Assume that a reaction will occur if this centrifugal barrier can be overcome. Thus, calculate the maximum impact parameter, which leads to orbiting of the colliding particles; e.g., at closest approach, the effective potential has to be zero. Then ...
Thermodynamic Investigation of the AINC and AICN Isomers by
... been determined by electrothermal atomic absorption spectrometry under the assumption that the observed decrease of absorption of elemental Al in the presence of nitrogen, N2, inside a graphite furnace at high temperatures ~e.g., 2700 K! in comparison with that in an argon atmosphere, to be solely d ...
... been determined by electrothermal atomic absorption spectrometry under the assumption that the observed decrease of absorption of elemental Al in the presence of nitrogen, N2, inside a graphite furnace at high temperatures ~e.g., 2700 K! in comparison with that in an argon atmosphere, to be solely d ...
Energetics of adsorption of neutral and charged molecules at the air
... group. Figure 4 shows the surface SH signal for a series ofpn-alkyl phenolate and anilinium ions at a fixed bulk concentration. In both cases, it can be seen that when the alkyl chain has five or more carbons, hydrophobicity overcomes solvation. Adsorption isotherms for phenolates and aniliniums wit ...
... group. Figure 4 shows the surface SH signal for a series ofpn-alkyl phenolate and anilinium ions at a fixed bulk concentration. In both cases, it can be seen that when the alkyl chain has five or more carbons, hydrophobicity overcomes solvation. Adsorption isotherms for phenolates and aniliniums wit ...
Protons, neutrons and electrons Isotopes Atomic mass units and
... Always put a plus charge on anything you suggest for a peak. Nothing can be detected in mass spectrometer without a plus-charge! ...
... Always put a plus charge on anything you suggest for a peak. Nothing can be detected in mass spectrometer without a plus-charge! ...
AP Chemistry Chapter 11 Notes - Properties of Solutions In a , or
... Psoln = vapor pressure of the solution χsolvent = mole fraction of the solvent P°solvent = vapor pressure of the pure solvent Sample Exercise 11.6 on page 524. Predict the vapor pressure of a solution prepared by mixing 35.0 g solid Na2SO4 (molar mass = 142.05 g/mol) with 175 g water at 25 ºC. The v ...
... Psoln = vapor pressure of the solution χsolvent = mole fraction of the solvent P°solvent = vapor pressure of the pure solvent Sample Exercise 11.6 on page 524. Predict the vapor pressure of a solution prepared by mixing 35.0 g solid Na2SO4 (molar mass = 142.05 g/mol) with 175 g water at 25 ºC. The v ...
Theory of Coordination Chemistry
... ligands. The sulfide groups are either two- or threecoordinated. A common motif features a four iron ions and four sulfide ions placed at the vertices of a cubane-type structure. 4Fe-4S clusters ...
... ligands. The sulfide groups are either two- or threecoordinated. A common motif features a four iron ions and four sulfide ions placed at the vertices of a cubane-type structure. 4Fe-4S clusters ...
Lecture 3 - Classification and Nomenclature
... ligands. The sulfide groups are either two- or threecoordinated. A common motif features a four iron ions and four sulfide ions placed at the vertices of a cubane-type structure. 4Fe-4S clusters ...
... ligands. The sulfide groups are either two- or threecoordinated. A common motif features a four iron ions and four sulfide ions placed at the vertices of a cubane-type structure. 4Fe-4S clusters ...
Comparison between Free and Immobilized Ion
... van der Waals interaction is very weak (see Table 1), the attractive part of the PMF is largely due to the water-mediated hydrophobic interaction. Therefore, these two contact minima are both hydrophobic in nature. It is known that the thermodynamics of the hydrophobic hydration is size dependent.28 ...
... van der Waals interaction is very weak (see Table 1), the attractive part of the PMF is largely due to the water-mediated hydrophobic interaction. Therefore, these two contact minima are both hydrophobic in nature. It is known that the thermodynamics of the hydrophobic hydration is size dependent.28 ...
Effect of Electric Field on the Mobility of Carboxyl
... could be observed by electrophoresis if the charge of the particle were so large relative to its size that the velocity required for escape from its ion atmosphere could be attained at relatively low field strength. Extraordinarily high charge-to-size ratios are feasible for carboxyl-terminated dend ...
... could be observed by electrophoresis if the charge of the particle were so large relative to its size that the velocity required for escape from its ion atmosphere could be attained at relatively low field strength. Extraordinarily high charge-to-size ratios are feasible for carboxyl-terminated dend ...
Multiplets in Polymer Gels. Rare Earth Metal Ions Luminescence Study
... ca. 15%. In methanol solutions under the same conditions the decay curves for the mixture Eu(NO3)3/Tb(NO3)3 are fairly close to that for Tb(NO3)3 alone. This suggests that in the gel some of the RE cations are held by the polymer network in close molecular-scale proximity to one another indicating o ...
... ca. 15%. In methanol solutions under the same conditions the decay curves for the mixture Eu(NO3)3/Tb(NO3)3 are fairly close to that for Tb(NO3)3 alone. This suggests that in the gel some of the RE cations are held by the polymer network in close molecular-scale proximity to one another indicating o ...
Hydroxyl Group of a Phosphorylated Ribose
... some viral satellite RNAs and are involved in RNA selfcleavage.2-4 The reaction catalyzed by hammerhead ribozymes is a phosphate ester hydrolysis that is thought to proceed via activation of a specific 2′-OH group by proton abstraction. The resultant 2′-alkoxide acts as a nucleophile, attacking the ...
... some viral satellite RNAs and are involved in RNA selfcleavage.2-4 The reaction catalyzed by hammerhead ribozymes is a phosphate ester hydrolysis that is thought to proceed via activation of a specific 2′-OH group by proton abstraction. The resultant 2′-alkoxide acts as a nucleophile, attacking the ...
Theory of Ion Exchange
... CA (log kd"log (kA/BQ)!log CB) is that CA CB/kA/B. Thus, even if CA CB, the dependence may not be linear if the selectivity coefRcient is very large. This feature of kd is shown as calculated examples in Figure 2. It can be seen that, if the selectivity coefRcient is low, kd falls linearly with the ...
... CA (log kd"log (kA/BQ)!log CB) is that CA CB/kA/B. Thus, even if CA CB, the dependence may not be linear if the selectivity coefRcient is very large. This feature of kd is shown as calculated examples in Figure 2. It can be seen that, if the selectivity coefRcient is low, kd falls linearly with the ...
Original powerpoint (~1.9 MB)
... A typical Ca2+ concentration in seawater is 0.010 M. Will the precipitation of Ca(OH)2 be complete from a seawater sample in which [OH-] is maintained at 0.040 M? Ksp of Ca(OH)2 is 5.5 x 10-6 Answer: Since the final [Ca2+] is 3.4 x 10-3 M, which is 34 % of 0.010 M, the precipitation is not ...
... A typical Ca2+ concentration in seawater is 0.010 M. Will the precipitation of Ca(OH)2 be complete from a seawater sample in which [OH-] is maintained at 0.040 M? Ksp of Ca(OH)2 is 5.5 x 10-6 Answer: Since the final [Ca2+] is 3.4 x 10-3 M, which is 34 % of 0.010 M, the precipitation is not ...
Uranyl Ion Complexes with Ammoniobenzoates as
... proved to be particularly important since it paved the way for the synthesis of polyrotaxanes and molecular necklaces involving metal ion coordination by functional groups located at both ends of the included species.4 Molecules comprising a single ammonium group may also be of interest for the buil ...
... proved to be particularly important since it paved the way for the synthesis of polyrotaxanes and molecular necklaces involving metal ion coordination by functional groups located at both ends of the included species.4 Molecules comprising a single ammonium group may also be of interest for the buil ...
Ion source

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.