Final Review Sheet Answers (the 6 page packet)
... 12. Is used to explain why the boiling point of HF is greater than the boiling point of HBr = (A) 14. Is used to explain the fact that the carbon-to-carbon bonds in benzene, C6H6, are identical = (D) Explain each of the following in terms of atomic and molecular structures and/or intermolecular forc ...
... 12. Is used to explain why the boiling point of HF is greater than the boiling point of HBr = (A) 14. Is used to explain the fact that the carbon-to-carbon bonds in benzene, C6H6, are identical = (D) Explain each of the following in terms of atomic and molecular structures and/or intermolecular forc ...
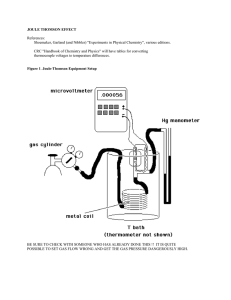
joule thomson effect
... µVvalues are proportional to ∆T, and therefore a plot of µV vs. kPa should be fairly linear. Before switching gases, make an Excel plot to see if the data is in fact reasonably linear. There may be a problem if there is significant curvature, and this should be ascertained before changing the gas. F ...
... µVvalues are proportional to ∆T, and therefore a plot of µV vs. kPa should be fairly linear. Before switching gases, make an Excel plot to see if the data is in fact reasonably linear. There may be a problem if there is significant curvature, and this should be ascertained before changing the gas. F ...
6.2 Solution Varieties
... into the air. The gas is CO2. ii. Adding Kinetic energy in the form of heat or shaking will increase the effervescence (by making the gas less soluble in the liquid). Gas molecules moving faster (greater Kinetic energy), makes it easier for them to escape the liquid’s attractive forces. C. Liquid in ...
... into the air. The gas is CO2. ii. Adding Kinetic energy in the form of heat or shaking will increase the effervescence (by making the gas less soluble in the liquid). Gas molecules moving faster (greater Kinetic energy), makes it easier for them to escape the liquid’s attractive forces. C. Liquid in ...
BAT
... g. When this reaction was carried out in the lab, 3.487 grams of product was obtained. What is the percent yield of this reaction? Chapter 10 Important topics: kinetic molecular theory, gas laws – Boyle, Charles, Gay-Lussac, combined, Dalton ...
... g. When this reaction was carried out in the lab, 3.487 grams of product was obtained. What is the percent yield of this reaction? Chapter 10 Important topics: kinetic molecular theory, gas laws – Boyle, Charles, Gay-Lussac, combined, Dalton ...
Metric conversion chart - Welcome to Chemistry At Central High
... Linear to Volume: When converting between linear and volume measurements, you need to cube the conversion factor! 10 cm = 1 dm : so (10 cm)3 = (1 dm)3 thus 1000 cm3 = 1 dm3 = 1 Liter Some Helpful Formulas: Gas Laws: Conversions: STP for gases = 0°C and 1 atm ; (or 273 K , 760 mm Hg) Combined : P1 V1 ...
... Linear to Volume: When converting between linear and volume measurements, you need to cube the conversion factor! 10 cm = 1 dm : so (10 cm)3 = (1 dm)3 thus 1000 cm3 = 1 dm3 = 1 Liter Some Helpful Formulas: Gas Laws: Conversions: STP for gases = 0°C and 1 atm ; (or 273 K , 760 mm Hg) Combined : P1 V1 ...
R= 8.31 J/mol K = 0.0821 L atm/mol K = 62.4 L torr/mol K PV = nRT
... ______15. How much heat is needed to raise the temperature of 1.00 mole of water from 10.0̊C to 50.0̊ C? A) 167 J B) 3.01 kJ C) 9.29 J D) 3760 J E) 40.0 kJ ______16. The pressure and kelvin temperature of a gas with a volume of 10.00 L are both doubled. The new volume of the gas is A) 10.00 L B) 2. ...
... ______15. How much heat is needed to raise the temperature of 1.00 mole of water from 10.0̊C to 50.0̊ C? A) 167 J B) 3.01 kJ C) 9.29 J D) 3760 J E) 40.0 kJ ______16. The pressure and kelvin temperature of a gas with a volume of 10.00 L are both doubled. The new volume of the gas is A) 10.00 L B) 2. ...
File
... ______15. How much heat is needed to raise the temperature of 1.00 mole of water from 10.0̊C to 50.0̊ C? A) 167 J B) 3.01 kJ C) 9.29 J D) 3760 J E) 40.0 kJ ______16. The pressure and kelvin temperature of a gas with a volume of 10.00 L are both doubled. The new volume of the gas is A) 10.00 L B) 2.5 ...
... ______15. How much heat is needed to raise the temperature of 1.00 mole of water from 10.0̊C to 50.0̊ C? A) 167 J B) 3.01 kJ C) 9.29 J D) 3760 J E) 40.0 kJ ______16. The pressure and kelvin temperature of a gas with a volume of 10.00 L are both doubled. The new volume of the gas is A) 10.00 L B) 2.5 ...
Gas Laws
... The ratio of the speeds of two gases at the same temperature is equal to the square root of the inverted molar masses The relative rate of diffusion Diffusion is the tendency of molecules to move toward areas of lower concentration to areas of higher concentration until the concentration is ...
... The ratio of the speeds of two gases at the same temperature is equal to the square root of the inverted molar masses The relative rate of diffusion Diffusion is the tendency of molecules to move toward areas of lower concentration to areas of higher concentration until the concentration is ...
Export To Word
... three state variables: absolute pressure (P), volume(V), and absolute temperature (T), and their relationship is explained with the help of kinetic theory. This virtual manipulative allows you to investigate various aspects of gases through virtual experimentation. From the site: Pump gas molecules ...
... three state variables: absolute pressure (P), volume(V), and absolute temperature (T), and their relationship is explained with the help of kinetic theory. This virtual manipulative allows you to investigate various aspects of gases through virtual experimentation. From the site: Pump gas molecules ...
IB 1 CHEMISTRY
... SI: the international system of measurment. The SI (Systeme International d´Unités) system has seven base units. All the other units are derived from them. The gram was originally defined in 1795 as the mass of one cubic centimeter of water at 4 °C, making the kilogram equal to the mass of one liter ...
... SI: the international system of measurment. The SI (Systeme International d´Unités) system has seven base units. All the other units are derived from them. The gram was originally defined in 1795 as the mass of one cubic centimeter of water at 4 °C, making the kilogram equal to the mass of one liter ...
File
... ___ 26) 50.0 milliliters. What volume will the gas have at a pressure of 20.0 kPa and a temperature of 200. K? ...
... ___ 26) 50.0 milliliters. What volume will the gas have at a pressure of 20.0 kPa and a temperature of 200. K? ...
OBL - USM
... Ideal gas law, Boyle’s Law, Charles’s Law, Avogadro’s hypothesis Gas mixtures, partial pressures, mole fractions Critical phenomena, compressibility factor, Z Van der Waals equation and other equations of state Elementary kinetic theory Maxwell – Boltzmann equation, probability density Types of aver ...
... Ideal gas law, Boyle’s Law, Charles’s Law, Avogadro’s hypothesis Gas mixtures, partial pressures, mole fractions Critical phenomena, compressibility factor, Z Van der Waals equation and other equations of state Elementary kinetic theory Maxwell – Boltzmann equation, probability density Types of aver ...
Problem solving techniques: comparing two cases
... heated from 200K to 300K. What is the new pressure at 300K? The ideal gas law applies: PV = nRT , but we don’t know either the volume or the number of moles of gas. So direct calculation of the pressure this way is not possible. Approach 1 : isolate the varying quantities, set the resulting expressi ...
... heated from 200K to 300K. What is the new pressure at 300K? The ideal gas law applies: PV = nRT , but we don’t know either the volume or the number of moles of gas. So direct calculation of the pressure this way is not possible. Approach 1 : isolate the varying quantities, set the resulting expressi ...
Review_WB_1
... 1. What volume will a sample of hydrogen occupy at 28.0 oC at 2.23 dm3 . What will the volume be at temperature of 0.0 oC? Assume that the pressure remains constant. (Remember Kelvin). P ...
... 1. What volume will a sample of hydrogen occupy at 28.0 oC at 2.23 dm3 . What will the volume be at temperature of 0.0 oC? Assume that the pressure remains constant. (Remember Kelvin). P ...
CHAPTER 5 REVIEW PACKET – GAS LAWS
... 18. Calculate the pressure that CCl4 will exert at 40.00C if 1.00 mol occupies 28.0L, assuming that (a) CCl4 obeys the ideal gas equation ...
... 18. Calculate the pressure that CCl4 will exert at 40.00C if 1.00 mol occupies 28.0L, assuming that (a) CCl4 obeys the ideal gas equation ...
R= 8.31 J/mol K = 0.0821 L atm/mol K = 62.4 L torr/mol K PV = nRT
... _____11. The RMS velocity of a gas varies A) directly with the molar mass of the gas B) inversely with the molar mass of the gas C) directly with the Kelvin temperature of the gas D) directly with the square root of the Kelvin temperature E) inversely with the square root of the Kelvin temperature. ...
... _____11. The RMS velocity of a gas varies A) directly with the molar mass of the gas B) inversely with the molar mass of the gas C) directly with the Kelvin temperature of the gas D) directly with the square root of the Kelvin temperature E) inversely with the square root of the Kelvin temperature. ...
Ch. 11: Gases
... 1) A gas is a collection of particles in constant, straight line motion. 2) Gas particles do not attract nor repel one another. They collide with each other and the walls of the container. 3) There is a lot of space between gas particles. 4) The average KE is proportional to the kelvin temperature o ...
... 1) A gas is a collection of particles in constant, straight line motion. 2) Gas particles do not attract nor repel one another. They collide with each other and the walls of the container. 3) There is a lot of space between gas particles. 4) The average KE is proportional to the kelvin temperature o ...
Lecture 5 (Slides Microsoft 97-2003) September 12
... • All gases exert pressure on the walls of their container. • The pressure exerted by a fixed amount of gas in a rigid container increases steadily as the gas temperature rises (Charles’s Law). • At a given T equal amounts (moles) of different gases with the same T and V exert the same pressure. ...
... • All gases exert pressure on the walls of their container. • The pressure exerted by a fixed amount of gas in a rigid container increases steadily as the gas temperature rises (Charles’s Law). • At a given T equal amounts (moles) of different gases with the same T and V exert the same pressure. ...
Gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture would contain a variety of pure gases much like the air. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer. The interaction of gas particles in the presence of electric and gravitational fields are considered negligible as indicated by the constant velocity vectors in the image. One type of commonly known gas is steam.The gaseous state of matter is found between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super cooled to incredibly low temperatures are classified by their statistical behavior as either a Bose gas or a Fermi gas. For a comprehensive listing of these exotic states of matter see list of states of matter.