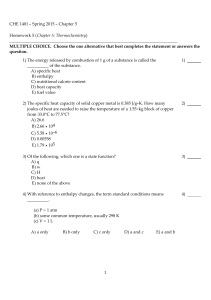
CHE 1401 - Fall 2015 - Chapter 5 Homework 5 (Chapter 5
... C) The ΔH for a process in the forward direction is equal to the ΔH for the process in the reverse direction. D) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps. E) The ΔH for a process in the forward ...
... C) The ΔH for a process in the forward direction is equal to the ΔH for the process in the reverse direction. D) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps. E) The ΔH for a process in the forward ...
CHE 1401 - Summer 2012 - Chapter 5 Homework 5 (Chapter 5
... 19) Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) The ΔH for a process in the forward direction is equal to the ΔH for the process in the reve ...
... 19) Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) The ΔH for a process in the forward direction is equal to the ΔH for the process in the reve ...
Thermodynamics
... An intensive property (also called a bulk property), is a physical property of a system that does not depend on the system size or the amount of material in the system. By contrast, an extensive property of a system does depend on the system size or the amount of material in the system. Examples for ...
... An intensive property (also called a bulk property), is a physical property of a system that does not depend on the system size or the amount of material in the system. By contrast, an extensive property of a system does depend on the system size or the amount of material in the system. Examples for ...
Document
... implies that there are no changes with time. The term uniform implies no change with location over a specified region. Engineering flow devices that operate for long periods of time under the same conditions are classified as steady-flow devices. The processes for these devices is called the steady- ...
... implies that there are no changes with time. The term uniform implies no change with location over a specified region. Engineering flow devices that operate for long periods of time under the same conditions are classified as steady-flow devices. The processes for these devices is called the steady- ...
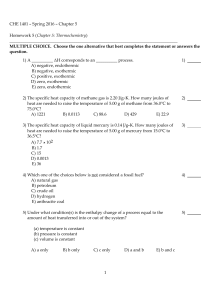
CHE 1401 - Spring 2015 - Chapter 5 Homework 5 (Chapter 5
... 14) Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to t ...
... 14) Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to t ...
TDDFT as a tool in chemistry and biochemistry
... small molecules, and light (or electromagnetic radiation)." […] Photochemistry may also be introduced to laymen as a reaction that proceeds with the absorption of light. Normally a reaction (not just a photochemical reaction) occurs when a molecule gains the necessary activation energy to undergo ch ...
... small molecules, and light (or electromagnetic radiation)." […] Photochemistry may also be introduced to laymen as a reaction that proceeds with the absorption of light. Normally a reaction (not just a photochemical reaction) occurs when a molecule gains the necessary activation energy to undergo ch ...
Corporate Profile
... • Isolated system – no exchange of matter or energy with the surroundings • Closed system – exchange of energy, but no exchange of matter with the surroundings • Open system – exchange of both energy and matter ...
... • Isolated system – no exchange of matter or energy with the surroundings • Closed system – exchange of energy, but no exchange of matter with the surroundings • Open system – exchange of both energy and matter ...
Complex Ions and Free Energy
... solution at equilibrium when concentrated ammonia is added to a 0.010 M solution of AgNO3 to give an equilibrium concentration of [NH3] = 0.20 M. Neglect the small volume change that occurs when NH3 is added ...
... solution at equilibrium when concentrated ammonia is added to a 0.010 M solution of AgNO3 to give an equilibrium concentration of [NH3] = 0.20 M. Neglect the small volume change that occurs when NH3 is added ...
Document
... A solid has a mass of 20g. When it is mixed with a solution a chemical reaction occurs in which a gas is produced. If the final total mass of the products is 55g, what was the mass of the solution? 20 g + solution = 55g 55g - 20g = mass of solution 35g = mass of solution ...
... A solid has a mass of 20g. When it is mixed with a solution a chemical reaction occurs in which a gas is produced. If the final total mass of the products is 55g, what was the mass of the solution? 20 g + solution = 55g 55g - 20g = mass of solution 35g = mass of solution ...
Solution - Georgetown Independent School District
... Internal Energy, Heat, and Work A balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 4.00 × 106 L to 4.50 × 106 L by the addition of 1.3 × 108 J of energy as heat. ...
... Internal Energy, Heat, and Work A balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 4.00 × 106 L to 4.50 × 106 L by the addition of 1.3 × 108 J of energy as heat. ...
Thermal Physics
... DU = Q - W DU : change in internal energy of system (J) Q: heat added to the system (J). This heat exchange is driven by temperature difference. W: work done on the system (J). Work will be related to the change in the system’s volume. This law is sometimes paraphrased as “you ...
... DU = Q - W DU : change in internal energy of system (J) Q: heat added to the system (J). This heat exchange is driven by temperature difference. W: work done on the system (J). Work will be related to the change in the system’s volume. This law is sometimes paraphrased as “you ...
Chemistry Final Test 1999-2000 - Nashoba Valley Technical High
... PREAPPROVED: 8.5” x 11” note sheet (Honors: 3” x 5” note card) which must be handwritten. Bring: # 2 pencils, eraser, something to read when you are done. In the following packet are the topics studied during the entire school year. This final exam is cumulative. Sample test questions are also inclu ...
... PREAPPROVED: 8.5” x 11” note sheet (Honors: 3” x 5” note card) which must be handwritten. Bring: # 2 pencils, eraser, something to read when you are done. In the following packet are the topics studied during the entire school year. This final exam is cumulative. Sample test questions are also inclu ...
2 - CronScience
... Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “precipitate” ...
... Ag1+ + NO31- + Na1+ + Cl1- AgCl + Na1+ + NO31Note that the AgCl did not ionize, because it is a “precipitate” ...
Learning Outcomes
... The total entropy change is normally expressed in terms of Gibbs free energy (G). The direction of spontaneous change is in the direction of decreasing free energy. The change in standard free energy for a reaction is related to the standard enthalpy and entropy changes by: ...
... The total entropy change is normally expressed in terms of Gibbs free energy (G). The direction of spontaneous change is in the direction of decreasing free energy. The change in standard free energy for a reaction is related to the standard enthalpy and entropy changes by: ...
Electrochemistry primer
... it or doing any other multiple) will result in the same electromotive force. The amount of charge transferred in a given electrochemical cell reaction is equal to nF where n is the moles of electrons transferred in the balanced cell reaction and F is Faraday’s constant. Thus the energy generated by ...
... it or doing any other multiple) will result in the same electromotive force. The amount of charge transferred in a given electrochemical cell reaction is equal to nF where n is the moles of electrons transferred in the balanced cell reaction and F is Faraday’s constant. Thus the energy generated by ...
PHYSICAL SETTING CHEMISTRY
... 51 Draw a Lewis electron-dot diagram for an atom of silicon. [1] Base your answers to questions 52 through 54 on the information below. ...
... 51 Draw a Lewis electron-dot diagram for an atom of silicon. [1] Base your answers to questions 52 through 54 on the information below. ...
Document
... greater the charge difference, the more energy is stored within the system. (c) Chemical - this is slightly more complex. Certain chemicals have bonds which require little energy to break. This energy must be put into the bond to break it. However, during the course of the chemical reaction, new bon ...
... greater the charge difference, the more energy is stored within the system. (c) Chemical - this is slightly more complex. Certain chemicals have bonds which require little energy to break. This energy must be put into the bond to break it. However, during the course of the chemical reaction, new bon ...
Section 01 Introduction to Analytical Chemistry ( powerpoint )
... techniques or principles for chemical measurements. • or • Conducts fundamental studies of chemical/physical phenomena underlying chemical measurements. ...
... techniques or principles for chemical measurements. • or • Conducts fundamental studies of chemical/physical phenomena underlying chemical measurements. ...
Chem 206 Exam 2 Answers
... d) After equilibrium is obtained, you add a catalyst and 3.00 additional moles of HCl. What will happen? <8 pts.> The addition of a catalyst will not change the equilibrium but will only increase the rate at which equilibrium will be obtained. The addition of HCl will cause the reaction to go to the ...
... d) After equilibrium is obtained, you add a catalyst and 3.00 additional moles of HCl. What will happen? <8 pts.> The addition of a catalyst will not change the equilibrium but will only increase the rate at which equilibrium will be obtained. The addition of HCl will cause the reaction to go to the ...
6. Absorption of Heat
... HRW 54E (5th ed.). A 150 g copper bowl contains 220 g of water, both at 20.0˚C. A very hot 300 g copper cylinder is dropped into the water, causing the water to boil, with 5.00 g being converted to steam. The final temperature of the system is 100˚C. (a) How much heat was transferred to the water? ...
... HRW 54E (5th ed.). A 150 g copper bowl contains 220 g of water, both at 20.0˚C. A very hot 300 g copper cylinder is dropped into the water, causing the water to boil, with 5.00 g being converted to steam. The final temperature of the system is 100˚C. (a) How much heat was transferred to the water? ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.