NAME: CHEMISTRY I CHAPTER 6 TYPES OF CHEMICAL
... CHEMISTRY I CHAPTER 6 TYPES OF CHEMICAL REACTIONS LABORATORY Most reactions can be classified into one of four major categories: ...
... CHEMISTRY I CHAPTER 6 TYPES OF CHEMICAL REACTIONS LABORATORY Most reactions can be classified into one of four major categories: ...
Chapter 3 Lecture Notes
... An important note about grams and AMU’s. Determing formula weights in either grams or amu’s are done in an identical manner. The difference is that if we are talking about the formula weight of an individual atom or molecule, then that mass unit is the amu. However, it is much more common, to be mo ...
... An important note about grams and AMU’s. Determing formula weights in either grams or amu’s are done in an identical manner. The difference is that if we are talking about the formula weight of an individual atom or molecule, then that mass unit is the amu. However, it is much more common, to be mo ...
Chemistry: Matter and Change
... two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. ...
... two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. ...
Chapter 7 – Chemical Reactions and Energy Flow
... So, heat is a measure of the flow of energy between the system and its surroundings. An object or solution or chemical compound does not contain heat. Rather, there is thermal energy within a system that can manifest as heat. Wood, for example, has less thermal energy than coal on a mass bases. But ...
... So, heat is a measure of the flow of energy between the system and its surroundings. An object or solution or chemical compound does not contain heat. Rather, there is thermal energy within a system that can manifest as heat. Wood, for example, has less thermal energy than coal on a mass bases. But ...
19A4B1A5.tmp - Louisiana Tech University
... 1. If DH is negative it helps product to be favored 2. If DS is positive it helps product to be favored 3. If DG is negative reaction is product favored Gibbs free energy change = difference between the enthalpy of a system and the product of its absolute temperature and entropy predictor of spontan ...
... 1. If DH is negative it helps product to be favored 2. If DS is positive it helps product to be favored 3. If DG is negative reaction is product favored Gibbs free energy change = difference between the enthalpy of a system and the product of its absolute temperature and entropy predictor of spontan ...
Press Release
... in High Temperature and Harsh Chemical Environments December 2006, Tinley Park, IL – PANDUIT introduces a high temperature and chemical resistant cable tie, ideal for applications where extreme temperatures (-75ºF to 500º F / 260º C to -59º C) or harsh chemical environments are the norm. PAN-TY® Cab ...
... in High Temperature and Harsh Chemical Environments December 2006, Tinley Park, IL – PANDUIT introduces a high temperature and chemical resistant cable tie, ideal for applications where extreme temperatures (-75ºF to 500º F / 260º C to -59º C) or harsh chemical environments are the norm. PAN-TY® Cab ...
Thermodynamic Laws, Entropy and CPH Theory
... the amount of heat absorbed in a reversible process in which the system goes from one state to another, and T is the absolute temperature.[3] Entropy is one of the factors that determines the free energy of the system. ...
... the amount of heat absorbed in a reversible process in which the system goes from one state to another, and T is the absolute temperature.[3] Entropy is one of the factors that determines the free energy of the system. ...
S.O.L. Review
... 4. Water can be made to boil above its normal boiling point of 100oC by – a. decreasing the air pressure b. increasing the air pressure c. increasing the heat being applied ...
... 4. Water can be made to boil above its normal boiling point of 100oC by – a. decreasing the air pressure b. increasing the air pressure c. increasing the heat being applied ...
Test review
... _____ 3. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. The total pressure is measured to be 9.0 atm. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? (A) It depends ...
... _____ 3. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. The total pressure is measured to be 9.0 atm. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? (A) It depends ...
our provided Word-Template - sCO2-Seminar-2016
... MgO / Mg(OH)2 (MgCO3). These materials show acceptable storage density of about 2 MJ/kg in case of using gaseous water for hydration, especially at MgO. In particular at hydration with steam the energy demand for evaporation has to be considered, because storage density could be halved if evaporatio ...
... MgO / Mg(OH)2 (MgCO3). These materials show acceptable storage density of about 2 MJ/kg in case of using gaseous water for hydration, especially at MgO. In particular at hydration with steam the energy demand for evaporation has to be considered, because storage density could be halved if evaporatio ...
Chapter6 - GEOCITIES.ws
... Conversions of different forms of energy are governed by : The Law of Conservation of Energy: Energy may be converted from one form to another, but the total quantity of energy remains constant. ...
... Conversions of different forms of energy are governed by : The Law of Conservation of Energy: Energy may be converted from one form to another, but the total quantity of energy remains constant. ...
C 3 H 8 (g) - Ms Critchley`s Lab
... 6. C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(l) 7. C2H4(g) + H2(g) C2H6(g) 8. 2C2H6(l) + 7O2(g) 4CO2(g) + 6H2O(l) Write the equation for ΔHc for 9. H2(g) + ½ O2(g) H2O(l) 10. CH3OH(l) + O2(g) CO2(g) + H2O(l) 11. C(s) + O2(g) CO2(g) ...
... 6. C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(l) 7. C2H4(g) + H2(g) C2H6(g) 8. 2C2H6(l) + 7O2(g) 4CO2(g) + 6H2O(l) Write the equation for ΔHc for 9. H2(g) + ½ O2(g) H2O(l) 10. CH3OH(l) + O2(g) CO2(g) + H2O(l) 11. C(s) + O2(g) CO2(g) ...
158KB - NZQA
... that the reaction lies to the products side as the larger the Kc or Q value, the greater the numerator (products). ...
... that the reaction lies to the products side as the larger the Kc or Q value, the greater the numerator (products). ...
27 Oct. 2010 - PHA Science
... 1.435 grams of naphthalene (C10H8) was burned in a constant-volume bomb calorimeter. The temperature of the water rose from 20.28oC to 25.95oC. If the heat capacity of the bomb plus water was 10.17 kJ/oC, calculate the heat of combustion of naphthalene on a molar basis (find the molar heat of combus ...
... 1.435 grams of naphthalene (C10H8) was burned in a constant-volume bomb calorimeter. The temperature of the water rose from 20.28oC to 25.95oC. If the heat capacity of the bomb plus water was 10.17 kJ/oC, calculate the heat of combustion of naphthalene on a molar basis (find the molar heat of combus ...
Reactions and Balancing
... Combustion reactions are the ones that burn (or explode!). There are two types of combustion reactions—complete or incomplete reactions. These reactions are identified by their products. They either produce carbon monoxide and water or carbon dioxide and water. ...
... Combustion reactions are the ones that burn (or explode!). There are two types of combustion reactions—complete or incomplete reactions. These reactions are identified by their products. They either produce carbon monoxide and water or carbon dioxide and water. ...
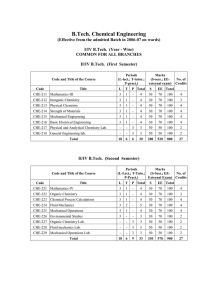
Chap-12A_Basic-Thermo-and-Laws
... – Property: characteristic of system such as temperature, pressure,… – State: condition of a system as described by its properties. • Any property change RESULTS in state changes – Process: a change in state (one or more properties change). • It is related to path followed – Extensive and intensive ...
... – Property: characteristic of system such as temperature, pressure,… – State: condition of a system as described by its properties. • Any property change RESULTS in state changes – Process: a change in state (one or more properties change). • It is related to path followed – Extensive and intensive ...
Chemistry - Nagpur University
... CH – 202: Paper- II ( Physical Chemistry) UNIT-I : Thermodynamics 7.5 Hrs (A) Recapitulation of thermodynamic terms : system, surrounding, types of system (closed, open & isolated), Thermodynamic, variables, intensive & extensive properties, thermodynamic processes isothermal, adiabatic, isobaric, i ...
... CH – 202: Paper- II ( Physical Chemistry) UNIT-I : Thermodynamics 7.5 Hrs (A) Recapitulation of thermodynamic terms : system, surrounding, types of system (closed, open & isolated), Thermodynamic, variables, intensive & extensive properties, thermodynamic processes isothermal, adiabatic, isobaric, i ...
1 Chemical Reactions and Equations
... (ii) Formulae of substances produced in the reaction i.e., products. (iii) The relative number of molecules of reactants and products. (iv) The relative masses of reactants and products. (v) The relative volumes of gaseous substances involved in the reaction. Q. 12. Enlist the limitations of chemica ...
... (ii) Formulae of substances produced in the reaction i.e., products. (iii) The relative number of molecules of reactants and products. (iv) The relative masses of reactants and products. (v) The relative volumes of gaseous substances involved in the reaction. Q. 12. Enlist the limitations of chemica ...
Rate and Equilibrium
... If the concentration of a substance involved in equilibrium is artificially increased, the reaction tends to adjust the composition so as to minimize the increase. Kc remains unchanged. Under constant temperature, any concentration changes on a system at equilibrium will result in adjustment of the ...
... If the concentration of a substance involved in equilibrium is artificially increased, the reaction tends to adjust the composition so as to minimize the increase. Kc remains unchanged. Under constant temperature, any concentration changes on a system at equilibrium will result in adjustment of the ...
Chem - Andhra University
... Heat changes, Enthalpy, reversible changes, maximum work. Heat capacities at constant pressure and volume, adiabatic changes. Heat of Reaction, heat of Formation, Heat of Combustion, Thermo-chemical Laws, effect of temperature on Heat of Reaction. Second law of Thermodynamics, spontaneous processes, ...
... Heat changes, Enthalpy, reversible changes, maximum work. Heat capacities at constant pressure and volume, adiabatic changes. Heat of Reaction, heat of Formation, Heat of Combustion, Thermo-chemical Laws, effect of temperature on Heat of Reaction. Second law of Thermodynamics, spontaneous processes, ...
CHEM230P1_06_2014_Y_P1
... system). The specific heat capacity of copper is 0.385 J K–1 g–1 and practically does not change over the temperature range involved. Show your reasoning clearly. ...
... system). The specific heat capacity of copper is 0.385 J K–1 g–1 and practically does not change over the temperature range involved. Show your reasoning clearly. ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.